Induction of Ia Antigens on Synovial Cells by Gamma Interferon
Article information
Abstract
Gamma interferon (IFN-γ) is a potent inducer of class II MHC antigens (la) in vitro. Enhanced Ia Expression is a characteristic feature of inflamed rheumatoid synovium. We investigated the potential of Ia induction upon non-inflammatory synovial cells under the influences of gamma interferon, alpha interferon and conditional medium from concanavalin A (Con-A) stimulated peripheral blood lymphocytes cultures (Con-A CM), Ia expression on enzyme dispersed cultured synovial cells decreased with time coincident with the loss of macrophage like cells.
Addition of gamma interferon induced Ia molecules upon Ia negative synovial cells in a time and dose dependent manner, whereas alpha interferon treatment failed to induced Ia antigen.
Con-A CM exerted the ability to induce Ia upon synovial cells. These findings suggest that: (1) Ia antigen expression by synovial tissue is the result of direct T cell influence: (2) non-inflammatory synovium has a potential to modulate Ia molecules under an altered environment.
INTRODUCTION
The rheumatoid synovium is characterized by (1) the proliferation of synoviocytes and (2) the infiltration of a large numbers of lymphocytes, especially activated T lymphocytes1,2). Interest in immunohistologic studies has been further increased by ample of reports documenting an association between class II MHC antigens and inflammatory joint diseases such as rheumatoid arthritis3,4). The characteristic enhanced expression of Ia antigen on rheumatoid synovium has also been documented5). These findings have indicated a potentially important role of synovial cells in la-dependent events, Ia antigen serves as a restriction element in antigen presentation to T lymphocytes and signal of activation6,7). Recent studies suggest that expression of Ia antigen upon various lines of cells, including endothelial cells, demal fibroblasts and a variety of neoplastic cell lines, can be induced by soluble products of activated T lymphocytes or gamma interferon 7–10). Recently Amento et al. showed expression of Ia antigen can be induced by gamma interferon in rheumatoid synovial cells11). However the latter study with gamma interferon has only focused on the effects of this compound on rheumatoid synovial cells which already showed enhanced association with Ia antigens. Thus we undertook this study to understand more fully the potential of synovial cells to interact with lymphokines by using non-inflammatory synovial cells which manifest a much lower degree of Ia expression than rheumatoid synovium in situ.
METHODS
1. Collection of Tissues
Sterile synovial tissue was obtained during open joint surgery from patients with non-inflammatory joint diseases including osteoarthritis or osteonecrosis. No patient was receiving gold, D-penicillamine or corticosteroid at the time of surgery.
2. Isolation & Cultrue of Synovial Cells
A portion of synovium was placed in OCT compound (Tissue Tek) and snap-frozen in liquid nitrogen for tissue sections. The fresh synovial tissues were repeatedly washed with sterile phosphate-buffered saline (PBS). Then the synovial membrane was dissected away and finely minced in Dulbecco’s modified Eagle’s (DME) media (Gibco). The tissue was mixed with 20 volumes of DMEM containing 1 mg/ml collagenase (Sigma) and 0.15 mg/ml DNase (Sigma) and stirred in a small spinner flask for 2–3 hours at 37°C until completely digested12). An equal volume of 0.025% trypsin was added and incubated for a further 30 min under the same conditions. Subsequently, the cell suspension was filtered through a nylon sieve (Tetco) with a pore sized of 80 μm. These cells were then washed three times. The pellet was resuspended in DMEM supplemented with 10% fetal calf serum (Gibco), 100 u/ml penicillin and 100 μg/ml streptomycin (Flow lab). The suspension was transferred to 60 mm plastic Petri dishes (Falcon) and left undisturbed at 37°c in a humidified chamber of 5% CO2. After overnight incubation the non-adherent floating cell population was removed. The adherent population was maintained in culture and passaged with trypsinization. For study, cells were plated on the third or fourth passage in 24-well trays (Costar) in 1 × 105 cells/well.
3. Preparation of Lymphocyte Conditional Media
Lymphocytes were separated from heparinized peripheral blood by density gradient centrifugation over Ficoll-Hypague (Bio-Rad) and then washed in PBS. The cell pellet was resuspended in RPMI 1640 (Gibco) with 10% fetal calf serum and incubated overnight at 37°C. After overnight incubation the non-adherent cell population was collected and readjusted to a final concentration of 2 × 106 cells/ml. This cell suspension was incubated at 37°C in the presence of 4 μg/ml of Con-A (Flow).
After 48 hour the supernatant was harvested after centrifugation and stored at −70°C until needed13).
4. Interferon
Affinity purified human gamma interferon (1×106 u/ml) and alpha interferon (1×106 u/ml) were purchased from Interferon Science, USA.
5. Immunohistologic Study
A sterile coverslip was cut to the appropriate size and placed into each well of a 24-well tissue culture plate. A 1 ml aliquots of the synovial cell suspensions containing 2×104 cells was added to each well. The plate was incubated at 37°c overnight until the synovial cells were fully attached to the coverslip. Then 50 μl of gamma, alpha interferon or Con-A CM in the appropriate dilutions or titers were added to each well. After an incubation of varying periods, the coverslip was washed in PBS and fixed in cold acetone11).
For indicrect immunofluorescence the coverslip was incubated sequentially in normal goat serum, monoclonal anti-HLA-DR (DAKO) or 22c6 (anti-la, a gift from Dr. Robert Winchester) antibody14) and FITC-conjugated goat anti-mouse IgG (DAKO). The coverslip was then examined using fluorescent microscopy (Nikon). For immunoper-oxidase staining an acetone fixed cryostat section was incubated with normal horse serum followed by primary monoclonal antibody and biotinylated horse anti-mouse IgG (Vector) sequentially. Endogenous peroxidase activity was blocked by incubating the slides in 0.3% hydrogen peroxide in PBS for 30 min, followed by an avidin-biotinylated complex incubation for 45 min. The sections were developed by amino-ethyl-carbazol (AEC) or 3, 3′-Diaminobenizidine (DAB) and counterstained5).
RESULTS
1. 1a Expression on Synovial Tissue
Non-inflammatory synovial tissue showed scattered areas of Ia antigen expression, both on synovial intima (Fig. 1A) and in subsynovium (Fig. 1B). In subsynovium. Ia expression was observed around blood vessels (Fig. 1C). In contrast rheumatoid synovium, with a broader layer of synoviocytes, expressed Ia antigen in nearly 100% of cells (Fig. 1D).
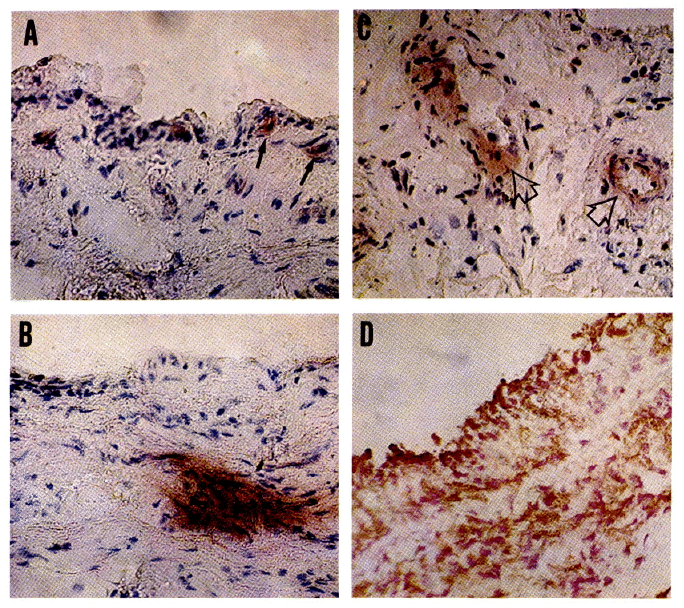
Morphologic appearance of non-inflamed synovial membrane after staining with anticodies to HLA-DR and Ia (22c6).
A. DR antigen positive cells are present in the synovial membrane and in the immediate neighbourhood (arrow) (×200).
B. Intensely DR antigen positive area in the subsynovial tissue (×200).
C. DR positive staining around blood vessels (arrow) (×200).
D. Rheumatoid synovial membrane exhibits a diffuse strong, positive reaction to 22c6 (Ia), both in the brodened layer of synoviocytes and in the subsynovium (Double configuration). Counterstained with nuclear fast red (×200).
2. Morphologic Study of Primary Culture of Adherent Synovial Cells
Cells from synovial tissue were readily dispersed using proteolytic enzymes. In the initial stage of culture, cells of varying morphology were present (Fig. 2A). Many small round cells were observed along with stellate cells and fibroblast like cells. The small round cells considered to be macrophages were positively stained with alpha-naphthyl acetate esterase (ANAE) (Fig. 2B & C). Passage of the primary cultures resulted in loss of the small round cells (Fig. 2D). In contrast spindle shaped fibroblast like cells became dorminate in the population. The large stellate cells persisted throughout the culture (Fig. 2E).

Photomicrograph of adherent synovial cells.
A. Phase contrast picture after initial dispersion. The stellate cells (S) and small round, macrophage like cells (M) are indicated by arrow (×100).
B–C. ANAE displaying small round cell (arrow) (×400).
D. Monolayer culture at second cell passage, Note the decrease in number of small round cells (×200).
E. The stellate cells with many branches of pseudopods and refractile granules in cytoplasm (×400).
3. Effect of Interferons and Con-A CM on Ia Expression
By the time of the third to fourth cell passage, regardless of cell type, la-positive cells were rarely seen. Therefore cells from the third or fouth passage were used for test. Treatment of a monolayer of synovial cells with gamma interferon reuslted in a dose dependent increase of Ia expression (Table 1). Maximal induction was observed with 100 u/ml gamma interferon, Ia expression and fluorescence intensity seemed to form a plateau at this concentration. The effect of gamma interferon was observed within 24 hours but required three to four days to become maximal. Some induction of Ia could be observed with a concentration as little as 1 u/ml of gamma interferon (Fig. 3A–3D).

Immunofluorescence micrograph showing induction of DR antigen expression on adherent synovial cells by gamma interferon (l00u/ml) for 3 days. Most of the synovial cells are DR positive (×400). Note DR negative cell with faint autofluorescence (arrow) in A.
In contrast, alpha interferon failed to induce Ia at any concentration in the range of 1–1000 μ/ml.
Con-A CM exerted a similar effect on Ia expression to gamma interferon. However, with a 10 vol % of Con-A CM, percentage of Ia inducible cells was not as high as that with gamma interferon.
DISCUSSION
This study was designed to investigate whether direct interactions between T lymphocyte products and synovial cells might influence the Ia activation potential of non-rheumatoid synovial cells. The ability of gamma interferon to induce Ia antigen in various cell lines including synovial cells from rheumatoid synovium has recently been described11).
Previously, expressions of Ia antigen on nearly 100% synovial cells of the tissue from patients with rheumatoid arthritis was demonstrated5).
In constrast Ia antigen expression upon synovial cells from normal, osteoarthritis, or traumatic arthritis is relatively quite low5). According to these findings it is speculated that enhanced Ia expression in rheumatoid synovium might be a secondary effect of infiltrating T lymphocytes11) However in non-inflammatory synovium we frequently find scattered areas of Ia expression without involvement of lymphocytic, infiltration
At present we are unable to correlate this finding as a result of T lymphocyte influence. Some of possible explanations for this scheme would be: (1) interstitial shedding of Ia molecule by a subpopulation of Ia bearing fibroblast-like cells: (2) activation of certain subsets of fibroblasts mediated through locally secreted soluble factors rather than gamma interferon11). A recent intriguing study, performed by Sobel et al., demonstrated enhanced endothelial Ia staining preceeding and during T cell mediated CNS injury in experimental allergic encephalitis (EAE)15). The finding suggests increased endothelial Ia is a proinflammatory, target organ-specific; alteration that persists during inflammation and may be part of the local immune response.
Our observations from cultures of non-inflammatory synovial cells agree with the previous presentation of Amento et al. that synovial fibroblast like cells upon initial isolation exhibited Ia antigens, the expression of which decreased with cell passage, coincident with the loss of monocytes and lymphocytes11,12).
The incubation of Ia negative cells with gamma interferon induced nearly all of the adherent synovial cells to become Ia positive. The mechanism of MHC antigen regulation in vascular endothelial cells and stromal fibroblast as reported by Collins et al., appears to induced HLA by transcription of apparently silent genes and subsequently increase mRNA in an expression of surface antigens16).
Alpha-interferon failed to induce la, but it shares the ability to induce class I MHC antigens upon target cells with T cell (gamma) and fibroblast (beta) interferon17). The crude preparation produced by Con-A stimulated peripheral blood lymphocytes, which may contain gamma interferon and other lymphokines, exerted a similar effect of Ia induction on synovial cells. However at higher concentrations it appeared to be a less effective inducer than purified gamma interferon. It is unclear whether this crude preparation contains any unknown factor inhibitory to Ia antigen induction18). Although the gamma interferon used in this study was affinity purified, the possibility of Ia gene activiation by other contaminating cytokines still exists and is yet to be investigated by applying recombinant gamma interferon.
A biological role for Ia expression is not clear in vivo, but observations that gamma interferon induces Ia molecule from synovial cells suggests that those modified cells could also participate in immune related events such as antigen presentation to T lymphocytes19,20), or yet unknown T lymphocytes-fibroblast interaction21–23).
In summary, we have shown that non-inflammatory synovial cells have the potential to express Ia antigen under the stimulation of gamma interferon or activated T lymphocyte product through a mechanism similar to that previously observed in rheumatoid synovial cells.
Acknowledgements
We thank In Sook An and Myung Hee Kim for technical assistance and Myung Hee Hong for preparation of the manuscript. This work supported in part by clinical Faculty Research Funds, Hanyang University Hospital.
