Influence of Pentobarbital-Na on Stimulation-Evoked Catecholamine Secretion in The Perfused Rat Adrenal Gland
Article information
Abstract
Objectives
The present study was attempted to investigate the effects of pentobarbital-Na, one of the barbiturates which are known to depress excitatory synaptic transmission in the central nervous system at concentrations similar to those required for the induction and maintenance of anesthesia, on catecholamines (CA) secretion evoked by cholinergic stimulation and membrane-depolarization from the isolated perfused rat adrenal gland, and to clarify the mechanism of its action.
Methods
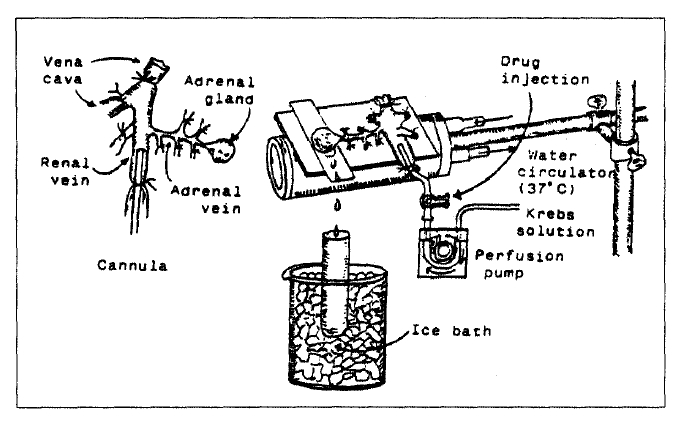
Mature male Sprague-Dawley rats were anesthetized with thiopenal-Na(40mg/kg, s.c.). The adrenal gland was isolated by the methods of Wakade. A cannula used for perfusion of the adrenal gland was inserted into the distal end of the renal vein. The adrenal gland was carefully removed from the animal and placed on a platform of a leucite chamber.
Results
The perfusion of pentobarbital-Na(30–300uM) into an adrenal vein for 20 min produced relatively dose-dependent inhibition in CA secretion evoked by ACh(5.32mM) DMPP(100uM for 1 min), McN-A-343(200uM for 2 min), Bay-K-8644(10uM) and high potassium(56mM), while it did not affect the CA secretion of cyclopiazonic acid(10uM). Also, in the presence of thiopental-Na (100uM), CA secretory responses evoked by ACh, DMPP, McN-A-343 and high K+ were markedly depressed. Moreover, in adrenal glands preloaded with ketamine(100uM for 20 min), which is known to be a dissociative anesthetic, CA secretion evoked by ACh, DMPP, McN-A-343 and high K+ were significantly attenuated.
Conclusion
Taken together, these experimental results suggest that pentobarbital-Na depresses CA release evoked by both cholinergic stimulation and membrane-depolarization from the isolated rat adrenal medulla and that this inhibitory activity may be due to the result of the direct inhibiton of Ca++ influx into the chromaffin cells without any effect on the calcium mobilization from the intracellular store.
INTRODUCTION
Many previous studies on pentobarbital have been directed to the effects of it on cardiac function, total peripheral resistance and arterial pressure1–5) since it is probably the most commonly used general anesthetic for cardiovascular, physiological and pharmacological studies in laboratory animals. Morita and his coworkers (1987)6) have suggested that steady-state effects of pentobarbital in intact rabbits are all due to baroreflex compensation; no change in mean arterial pressure or renal nerve activity. It is found that concentrations of pentobarbital, which are considered to be anesthetic, selectively block the fast excitatory postsynaptic potential and also indicate that blockade of sympathetic ganglionic transmission of the lumbar chain of frogs is solely mediated by a postsynaptic action7). Nicoll (1977)8) also reported that pentobarbital anesthesia results from a postsynaptic blockade of central excitatory synapses which increase sodium conductance coupled with a postsynaptic enhancement of gamma-amino-butyric acid-mediated synaptic inhibition. Barbiturates are known to reduce voltage-dependent calcium conductance by enhancing calcium channel inactivation or by producing open channel block of calcium channels9). Moreover, recently Sim and Radhakrishnan (1994),10) have shown that pentobarbital and chlordiazepoxide, administered intracerebroventricularly, attenuated dose-dependently the pressor action of intracerebroventricular angiotensin II and angiotensin III in the conscious spontaneously hypertensive rat and the normotensive control Wistar Kyoto rat. Potashner and his coworkers (1980)11) have found that calcium-dependent stimulus-evoked release of the putative excitatory transmitter glutamate is depressed by pentobarbital.
The adrenal chromaffin cell offers a suitable experimental model for such analysis as the mechanisms of transmitter secretion are well characterized12). In addition, it is already known from studies on whole glands that pentobarbital inhibits the secretion of catecholamines (CA) from the perfused isolated cow adrenal gland that is induced by nicotinic agonists and high K+13). It has been also demonstrated in the peripheral cholinergic synapses that halothane and other anesthetics depress the postsynaptic responses to the nicotinic actions of acetylcholine (ACh), whereas muscarinic actions are not affected14–18). Pocock and Richards (1987)19) have found that pentobarbital inhibits the CA secretion from bovine isolated adrenal chromaffin cells induced by nicotinic agonists and potassium-depolarization. Activation of the nicotinic receptors on the surface of the chromaffin cell causes inward movement of Na+ and possibly Ca++ ions. The resultant depolarization opens voltage-sensitive channels to bring about a rise in intracellular free Ca++ which triggers granule exocytosis20). Exposure of cells to high K+ bypasses the ACh receptor and elicits secretion by opening the voltage-sensitive Ca++ channels directly21). Therefore, the present study was made to investigate the action of pentobarbital on CA secretion evoked by both pathways, which are membrane-depolarization and cholinergic stimulation, and on the relationship between intracellular free Ca++ and exocytosis in the isolated perfused rat adrenal medulla.
MATERIALS AND METHODS
1. Experimental Procedure
Male Sprague-Dawley rats, weighing 180 to 250 grams, were anesthetized with thiopental sodium (40mg/kg) intraperitoneally. The adrenal gland was isolated by the methods described previously22). The abdomen was opened by a mid-line incision, and the left adrenal gland and surrounding area were exposed by placing three hook retractors. The stomach, intestine and portion of the liver were not removed, but pushed over to the right side and covered by saline-soaked gauge pads, and urine in the bladder was removed in order to obtain enough working space for tying blood vessels and cannulations.
As shown in Fig. 1, a cannula used for perfusion of the adrenal gland was inserted into the distal end of the renal vein after all branches of adrenal vein(if any), vena cava and aorta were ligated. Heparin (400IU/ml) was injected into vena cava to prevent blood coagulation before ligating vessels and cannulations. A small slit was made into the adrenal cortex just opposite to the entrance of the adrenal vein. Perfusion of the gland was started, after making sure that no leakage was present, and the perfusion fluid escaped only from the slit made in the adrenal cortex. Then the adrenal gland, along with ligated blood vessels and the cannula, was carefully removed from the animal and placed on a platform of a leucite chamber. The chamber was continuously circulated with water heated at 37±1°C.
2. Perfusion of Adrenal Gland
The adrenal glands were perfused by means of a ISCO pump (WIZ Co.) at a rate of 0.3ml/min. The perfusion was carried out with Krebs-bicarbonate solution of the following composition (mM): NaCl, 118.4; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.18; NaHCO3, 25; KH2PO4, 1.2; glucose, 11.7. The solution was constantly bubbled with 95% O2+5% CO2 and the final pH of the solution was adjusted to 7.4±0.1. The solution contained disodium EDTA (10ug/ml) and ascorbic acid (100ug/ml) to prevent oxidation of catecholamine.
3. Drug Administration
The perfusions of DMPP (100uM) for 1 min and McN-A-343 (100uM) for 2 minutes and/or a single injection of ACh (5.32mM) and KCl (56mM) in a volume of 0.05ml were made into the perfusion stream via a three-way stopcock, and Bay-K-8644 (10−5M) and cyclopiazonic acid (10−5M) were perfused for 4 min, respectively.
In the preliminary experiments, it was found that secretory responses to ACh, KCl, McN-A-343 and Bay-K-8644 returned to preinjection level in about 4 min, but the responses to DMPP in 8 min. In the present experiment, the adrenal glands were perfused with normal Krebs solution for about one hour before the experimental protocols were initiated.
4. Collection of Perfusate
As a rule, prior to each stimulation with cholinergic agonists or excess K+, perfusate was collected in a chilled tube for 4 min to determine the spontaneous secretion of CA (background sample). Immediately after the collection of the backgound sample, collection of the perfusates was continued in another tube as soon as the perfusion medium containing the stimulatory agent reached the adrenal gland. Stimulated samples were collected one or two times at intervals of 4 min. The amounts secreted in the background sample have been subtracted from those secreted from the stimulated sample to obtain the net secretion value of CA, which is shown in all of the figures and tables.
To study the effects of pentobarbital and other anesthetics on the spontaneous and evoked secretion, the adrenal gland was perfused with Krebs solution containing pentobarbital or other anesthetics for 20 min, then the perfusate was collected for a specific time(“background sample”), and then the medium was changed to the one containing the stimulating agent and the perfusates were collected for the same period as that for the background sample.
5. Measurement of Catecholamines
The CA content of perfusate was measured directly by the flurometric method of Anton and Sayre (1962)23) without the intermediate purification alumina for the reasons described earlier22) using fluorospectrophotometer (Shimadzu Co. Japan).
A volume of 0.2ml of the perfusate was used for the reaction. The CA content in the perfusate of stimulated glands by secretogogues used in the present work was high enough to obtain readings several fold greater than the reading of control samples (unstimulated). The sample blanks were also lowest for perfusates of stimulated and non-stimulated samples. The content of CA in the perfusate was expressed in terms of norepinephrine (base) equivalents.
6. Statistical Analysis
All data are presented as means with their standard errors, and the significance of differences was analyzed by Student’s paired t-test using the computer program as previously described24).
7. Drugs and Their Sources
The following drugs were used: Pentobarbital sodium (Tokyo Kasei Co., Ltd., Japan), acetylcholine chloride, 1.1-dimethyl-4-phenyl piperazinium iodide [DMPP], norepinephrine bitartrate, methyl-1, 4-dihydro-2, 6-dimethyl-3-nitro-4-(2-trifluoromethylphenyl)-pyridine-5-carboxylate [BAY-K-8644] (Sigma Chemical Co., U.S.A.), cyclopiazonic acid, [3-(m-cholro-phenyl-carbamoyl-oxy]-2-butynyl trimethyl ammonium chloride [McN-A-343] (RBI, U.S.A.). ketamine hydrochloride (Yuhan Co., Korea), thiopental sodium (Abbott, U.S.A.). Drugs were dissolved in distilled water (stock) and diluted with the normal Krebs solution except Bay-K-8644. Bay-K-8644 was dissolved in 99.5% ethanol and diluted appropriately with Krebs solution (final concentration of alcohol was less than 0.1%). Concentrations of all drugs used are expressed in terms of molar base.
RESULTS
1. Effect of 30uM pentobarbital-Na on CA secretion evoked by ACh, McN-A-343, excess K+ and DMPP from the isolated rat adrenal glands
After the initial perfusion with oxygenated Krebs-bicarbonate solution for 1 hr, basal CA release from the isolated perfused rat adrenal glands amounted to 22.5±2.4ng/2min (n=12). It was decided initially to examine the effects of pentobarbital, which is a typical and widely used intravenous anesthetic, on cholinergic receptor stimulation- as well as membrane depolarization-mediated CA secretion from perfused rat adrenal glands. Secretagogues were given at 20 to 30 min intervals. Pentobarbital was present 20 min before each stimulation. In the present study, it was found that pentobarbital itself did not produce any effect on basal CA output (data not shown).
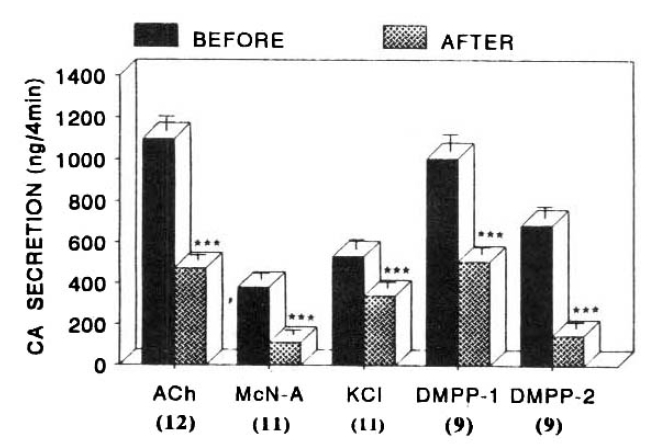
When ACh (5.32mM) in a volume of 0.05ml was injected into the perfusion stream, the amounts of CA secreted was 1035±112ng for 4min. However, after the preperfusion with 30 uM pentobarbital for 20 min, ACh-stimulated CA secretion was significantly decreased to 751 ±40ng (p<0.01,) for 4 min from 12 adrenal glands as shown in Fig. 2. As illustrated in Fig. 2, McN-A-343 (100uM), which is a selective muscarinic M1-agonist25), perfused into an adrenal gland for 2 min caused an increased CA secretion to 293±63ng for 4 min from 8 experiments. However, McN-A-343-stimulated CA secretion in the presence of 30uM pentobarbital was markedly inhibited to 174±34ng (p<0.05) for 4 min, which was 59% of the corresponding control secretion(Fig. 2).
Influence of 30uM pentobarbital-Na on nicotinic, muscarinic stimulation- and membrane depolarization-induced catecholamine (CA) secretory responses from the isolated perfused rat adrenal glands. CA secretion was induced by a single injection of ACh (5.32mM) and excess K+ (56mM), and by the perfusion of McN-A-343 (200uM) and DMPP (100uM) for 1 min, respectively, after perfusion with normal Krebs solution for one hour prior to initiation of the experimental protocol. “BEFORE” and “AFTER” denote CA secretion evoked by ACh, excess K, McN-A-343 and DMPP before and after preloading with 30 uM pentobarbital-Na for 20 min, respectively. Numbers in the parenthesis indicate number of experimental rat adrenal glands. Vertical bars represent the standard error of the mean (S.E.M.). Ordinate the amounts of CA secreted from the adrenal gland in ng. Abscissa: secretogogues. Statistical difference was obtained by comparing the control with the pretreated group. Each perfusate was collected for 4 minutes, but DMPP-induced perfusates was collected twice successively for each 4 minutes. ACh: acetylcholine. McN-A: McN-A-343. *:p<0.05, ***:p<0.01.
Interestingly, it has been found that a depolarizing agent like KCl stimulates sharply CA secretion but, in the present work, excess K+ (56 mM)-stimulated CA secretion after the pretreatment with 30uM pentobarbital for 20 min was also attenuated. In the presence of 30uM pentobarbital, it amounted to 400±45ng(p<0.05) for 4 min as compared with its corresponding control secretion of 608±834ng for 4 min from 6 glands(Fig. 2).
When perfused into the rat adrenal gland, DMPP (100uM for 1min), which is a selective nicotinic receptor agonist in autonomic sympathetic ganglia, evoked a sharp and rapid increase in CA secretion. As shown in Fig. 2, DMPP-stimulated CA secretion before preloading with 30 uM pentobarbital was 1175±185ng(0–4 min) and 808±94ng (4–8 min), while after pretreatment with 30 uM pentobarbital for 20 min they were greatly reduced to 975±137ng (0–4 min, p<0.05) and 440±48ng (4–8 min, p<0.05), respectively, from 6 rat adrenal glands.
2. Effect of 100uM pentobarbital-Na on CA secretion evoked by ACh, McN-A-343, excess K+ and DMPP from the isolated rat adrenal glands
In order to test the dose-dependent effects of pentobarbital on cholinergic receptor-stimulated CA secretion as well as membrane depolarization-mediated secretion, more increased concentration of pentobarbital to 100uM was preloaded into the adrenal medulla. Fig. 3 shows that 100uM pentobarbital-pretreatment exerts greatly inhibition of CA secretion evoked by ACh, excess K+, McN-A-343 and DMPP. In the present study, ACh (5.32mM)-stimulated CA secretion prior to preloading with 100uM pentobarbital was 912±71ng for 4 min from 9 rats. However, under 100uM pentobarbital effect, which was perfused 20 min before the stimulation was induced, it was markedly inhibited to 513±41ng (p<0.01) for 4 min, which was 56% of its corresponding control secretion (Fig. 3). In 5 rat adrenal glands, McN-A-343 (100 uM)-stimulated CA secretion was 276±23ng/4 min before administration of 100uM pentobarbital, but in the presence of 100uM pentobarbital, McN-A-343-evoked CA secretion was significantly decreased to 162±24ng (p<0.01) of its control secretion as shown in Fig. 3.
Excess K+ (56mM)-stimulated CA secretion in the presence of 100uM pentobarbital amounted to 330±22ng for 4 min from 8 rat glands as compared to the corresponding control secretion of 636±85ng/4min. There was significant difference (p<0.01) between the both groups before and after pretreatment with 100uM pentobarbital as shown in Fig. 3. Nicotinic receptor agonist, DMPP (100uM) perfused into the adrenal gland, evoked great CA secretion of 1016±108ng (0–4 min) and 501±94ng (4–8 min), while following perfusion with 100uM pentobarbital for 20 min they were markedly reduced to 720±92ng (0–4 min, p<0.01) and 182±49ng (4–8 min, p<0.01) from 7 adrenal glands as compared with their control responses, respectively, as shown as in Fig. 3.
3. Effect of 300uM pentobarbital on CA secretion evoked by ACh, McN-A-343, excess K+ and DMPP from the isolated rat adrenal glands
We tried to examine the effects of pentobarbital as a maximal concentration in the present experiment on cholinergic receptor-stimulated as well as membrane depolarization-mediated CA secretion from the isolated perfused rat adrenal glands. Prior to preloading with 300uM pentobarbital, CA secretion evoked by a single injection of ACh (5.32mM) in a volume of 0.05ml and by the perfusion of McN-A-343 into an adrenal gland amounted to 1098±69ng and 380±30ng for 4 min, respectively, as shown as in Fig. 6 and Table 3. However, following the preloading with 300uM pentobarbital for 20 min they were greatly blocked to 474±25ng (P<0.01, n=12) for 4 min and 114±24ng (p<0.01, n=11) compared to its corresponding control secretion (Fig. 4).
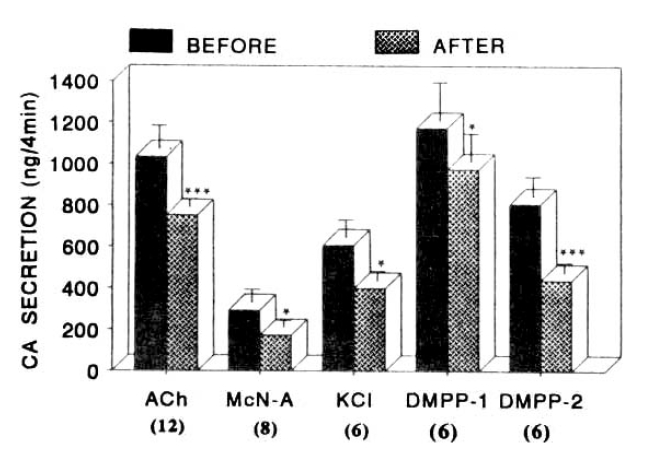
Influence of 100uM thiopental-Na on nicotinic, muscarinic stimulation- and membrane depolarization-induced catecholamine (CA) secretory responses from the isolated perfused rat adrenal glands. ACh (5.32mM), excess K (56mM), McN-A-343 (200uM) and DMPP (100uM) were introduced before and after preloading with 100uM thiopental-Na for 20 min, respectively. Other legends are the same as in Fig. 2. ***: p<0.01.
Fig. 4 shows the inhibitory effect of 300uM pentobarbital to CA secretory effect evoked by ACh, excess K+ McN-A-343 and DMPP from the rat adrenal glands. In the present work, in the absence of 300uM pentobarbital, DMPP (100uM)- and Excess K+ (56mM)-evoked CA secretion amounted to 1007±79ng (0–4 min) and 687±54ng (4–8 min), and 532±46ng (0–4 min), respectively, while in the presence of 300uM pentobarbital which was preloaded 20 min before stimulation, they were prominently depressed to 508±34ng (0–4 min, p<0.01, n=9) and 148±29ng (4–8 min, p<0.01, n=9) and 341±29ng (0–4 min, p<0.01, n=11), respectively, as shown in Fig. 4.
4. Effect of pentobarbital on cyclopiazonic acid- and Bay-K-8644-evoked CA secretion from the perfused rat adrenal glands
Since cyclopiazonic acid, a mycotoxin from Aspergillus and Penicillium, has been described as a highly selective inhibitor of Ca++-ATPase in skeletal muscle sarcoplasmic reticulum26–27) and it may be extremely valuable pharmacological tool for investigating intracellular Ca++ mobilization and ionic current regulated by intracellular calcium28), it is of particular interest to test the effect of pentobarbital on cyclopiazonic acid-induced CA secretory responses.
Fig. 5 shows the influence of pentobarbital on cyclopiazonic acid- and Bay-K-8644-evoked CA secretory response from the isolated rat adrenal glands. In the presence of 100 uM pentobarbital, cyclopiazonic acid (10uM) given into the adrenal gland for 4 min caused the CA secretory response of 188±13ng (ns) as compared to the corresponding control response of 197±14ng from 9 experiments.

Influence of 100uM pentobarbital-Na on CA secretion evoked by Bay-K-8644 and cyclopiazonic acid. Bay-K-8644 (Bay-K, 10 uM) and cyclopiazonic acid (CPA, 10uM) were perfused into an adrenal vein for 4 min before and after the preloading with 100uM pentobarbital-Na for 4 min before and after the preloading with 100uM pentobarbital-Na for 20 min. Its pefusate was collected for 4 min. Other legends are the same as in Fig. 2. ***: p<0.01.
Bay-K-8644 is known to be a calcium channel activator and to cause positive inotropy and vasconstriction in isolated tissues and intact animals29–30) and to enhance basal Ca++ uptake31) and CA release32). Therefore, it was likely of interest to determine the effects of pentobarbital on Bay-K-8644-stimulated CA secretion from the isolated perfused rat adrenal glands. As illustrated in Fig. 5, the inhibitory effect of 100 uM pentobarbital on Bay-K-8644-evoked CA secretion was observed. Bay-K-8644 (10uM) given into the perfusion stream for 4 min increased CA secretion to 833±65ng from 8 rat adrenal glands. However, under the effect of 100uM pentobarbital which was preloaded 20 min before the perfusion of Bay-K-8644, Bay-K-8644-stimulated CA secretion was strikingly depressed to 219±16ng (p<0.01) for 4 min as compared to the corresponding control release; thus, the release was reduced to 26% of the control secretion (Fig. 5).
6. Effect of thiopental on CA secretion evoked by ACh, McN-A-343, excess K+ and DMPP from the isolated rat adrenal glands
In the previous experimental results as shown in Fig. 2–7, it was found that pentobarbital showed relatively a dose-dependent inhibition in CA secretory responses evoked by nicotinic and muscarinic cholinergic stimulation and membrane depolarization. Moreover, it has been known that administration of thiopental depresses CA release evoked by ACh from the dog adrenal medulla17). Therefore, it is likely of great interest to examine the effect of thiopental on CA secretion evoked by various secretagogues.
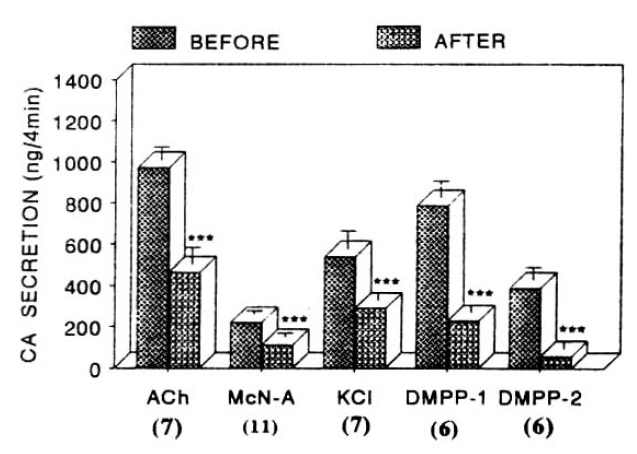
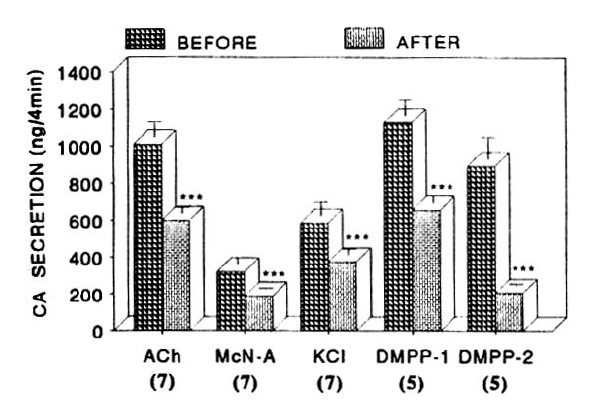
Influence of 100uM ketamine on nicotinic, muscarinic stimulation- and membrane depolarization-induced catecholamine (CA) secretory responses from the isolated perfused rat adrenal glands. ACh (5.32mM), excess K (56 mM), McN-A-343 (200uM) and DMPP (100 uM) were introduced before and after preloading with 100uM ketamine for 20 min, respectively. Other legends are the same as in Fig. 2. ***: p<0.01
CA release evoked by ACh (5.32mM) and McN-A-343 (100uM) after preloading with 100 uM thiopental for 20 min amounted to 600±33ng (p<0.01, n=7) and 189±5ng (p<0.01, n=7) for 4 min, respectively, as compared to each corresponding control secretion of 1009±80ng and 322±38ng for 4 min as shown in Fig. 6.
DMPP (100uM)- and excess K+ (56mM)-stimulated CA releases in the absence of thiopental were 1128±85ng (0–4 min) and 894±117ng (4–8 min), and 587±76ng (0–4 min), respectively. However, after preloading with 100uM thiopental for 20 min, they were significantly reduced to 657±39ng (0–4 min, p<0.01, n=5) and 207±11ng (4–8 min, p<0.01, n=5), and 375±32 (0–4 min, p<0.01, n=7), respectively, as compared to their corresponding control secretion as shown in Fig. 6.
7. Effect of ketamine on CA release evoked by ACh, McN-A-343, excess K+ and DMPP from the isolated rat adrenal glands
Since it has been found that administration of ketamine, which is known to be a dissociative anesthetic, inhibits CA release evoked by ACh from the dog adrenal medulla15, 17), it was tried to determine the effect of ketamine on CA secretion evoked by various secretagogues from the isolated rat adrenal glands.
When given into a adrenal vein, ACh (5.32mM)- and McN-A-343 (100uM)-induced CA, released in the presence of 100uM ketamine for 20 min, were considerably attenuated to 465±82ng (p<0.01, n=7) and 111±20ng (p<0.01, n=11), respectively, as compared to their control secretory responses of 971±63ng and 221±18ng as shown in Fig. 7.
On the other hand, ketamine treatment itself did fail to alter the basal CA secretory response (data not shown). As depicted in figure 12, DMPP (100 uM)- and excess K+ (56mM)-induced CA secretions under existence of 100 uM ketamine were also significantly inhibited to 228±40ng (0–4 min, p<0.01, n=6) and 58±34ng (4–8 min, p<0.01, n=6) and 289±39ng (0–4 min, p<0.01, n=7) of their corresponding control responses, respectively as compared to the corresponding control secretory responses of 788±63ng (0–4 min) and 390±62ng (4–8 min), and 540±87 (0–4 min) as depicted in Fig. 7.
DISCUSSION
The main object of the present study was to investigate the mechanism by which pentobarbital influences CA secretion using the isolated perfused rat adrenal gland as a model and to clarify the action of pentobarbital on extracellular and intracellular calcium mobilization. It has been demonstrated in the present investigation that the pentobarbital did inhibit dose-dependently cholinergic(nicotinic and muscarinic) receptor-stimulated and membrane depolarization-mediated CA secretory responses, as well as Bay-K-8644-induced CA release. However, cyclopiazonic acid-evoked CA secretion was not affected by the pretreatment with pentobarbital. The present experimental results, taken together, suggest strongly that pentobarbital inhibits CA secretion evoked by both cholinergic stimulation and membrane-depolarization, and that this inhibition may be due to the result of the direct inhibition of Ca++ influx into the chromaffin cells without any effect on the calcium mobilization from the intracellular store.
These results are consistent with the previous findings that this anesthetic decreases the secretion induced by carbachol and elevated concentration of K+19).
This pentobarbital-induced inhibitory responses of CA release was produced at concentrations (50–300uM) within the range required for the induction and maintenance of general anesthesia33) and, at these concentrations, pentobarbital did not affect the basal secretion of CA. Based on these investigational data, pentobarbital could interfere with CA release by 1) acting as receptor blocking agents, 2) stabilization of the membrane in a way that prevents a necessary configurational alteration, and 3) blockade of Ca++ influx or mobilization.
However, it seems that this inhibitory effect of pentobarbital is not due to receptor blockade, since pentobarbital blocks the effect of substances which stimulate CA release by stimulation of cholinergic (both nicotinic and muscarinic) receptors but also the effects of high potassium and Bay-K-8644. In support of these findings, Holmes and Schneider(1973)13) observed a similar response in the perfused isolated cow adrenal gland to that in the present study. Moreover, this is in agreement with the suggestion made over 20 years ago by Eccles (1946)34) that barbiturates act by increasing the stability of the nerve cell membrane to electrical changes. Sumikawa (1983)17) found that all of the anesthetics tested (alphaxalone, ketamine and thiopental), except for diazepam, caused marked inhibitory effects on the nicotinic response at clinical concentrations, while muscarinic responses were affected slightly or not at all from the dog adrenal medulla. A similar selectivity of actions of halothane14, 16) and ketamine15) have been demonstrated previously in the sympathetic ganglia, although clinical concentrations always were not used, in addition, it has been demonstrated that in the sympathetic ganglia of the lumbar chain of frogs, pentobarbital blocks the fast excitatory postsynaptic potential, and also its blockade of ganglionic transmission is solely mediated by a postsynaptic action7). However, it seems that there is some difference between the previous and the present experimental results. In the present experiments, pentobarbital inhibited the CA secretory responses evoked by DMPP and McN-A-343. This finding indicates that pentobarbital can block both nicotinic and muscarinic receptors in the rat adrenal medulla. On the other hand, this fact suggests strongly that the differing effects on the muscarinic responses might be one of factors involved in the specific properties of each anesthetic, whereas the inhibition of nicotinic responses might contribute to the common properties of anesthetics. In support of this finding, in contrast with the other anesthetics tested, diazepam at even anesthetic concentrations had no effect on either the nicotinic or muscarinic responses17). It could not be ruled out that there is a difference between species of animals in CA release from the adrenal medulla. In general, general anesthetics are known to possess several effects on synaptic transmission and physical state of the membranes. They exert various actions at the synapses, affecting both the amout of transmitter released35–36) and the sensitivity of the postsynatic membrane to the transmitter37–38). Moreover, it is conceivable that different synapses have varying degrees of stability and susceptibility to anesthetics39). Alper and his coworkers (1969)14) have demonstrated that halothane alone dose not affect the response to injected acetylcholine while it specifically inhibits the response of nicotinic ganglionic receptors alone, using cardiac sympathetic ganglia of dogs. On the other hand, Krnjevic (1975)40) has suggested that general anesthetics exert their action by blocking muscarinic excitation of the cortical neurons. On the contrary, using the olfactory cortex maintained in vitro, Smaje (1976)38) found that volatile anesthetics, such as halothane, caused a dose-related augmentation of muscarinic excitation. Based on these facts, it is thought that there are differences between organs and/or animal species in characteristics of selectivity by anesthetics to the cholinergic receptors-mediated CA release from the adrenal medulla. Moreover, in the present study, ketamine, as well as thiopental, greatly depressed CA secretory response evoked by both cholinergic receptor stimulation and membrane-depolarization. Thses results are very similar to that evoked by pentobarbital. In support of this idea, it has been found that administration of thiopental depresses CA release evoked by ACh from the dog adrenal medulla17), and that administration of ketamine, which is known to be a dissociative anesthetic, inhibits CA release evoked by ACh from the dog adrenal medulla15, 17). Thses findings indicate strongly that pentobarbital depresses CA secretory responses, evoked by nicotinic and muscarinic cholinergic receptors stimulation, as well as that by membrane depolarization in the isolated perfused rat adrenal gland, and suggest strongly that this inhibitory effect of pentobarbital is exerted by the suppression of Ca++ entry through receptor-linked and/or voltage-dependent Ca++ channels. The increase in secretion of CA that results from stimulation of the cells with carbachol or high K+ is associated with an increase in Ca++ influx41). It has been also found that the pentobarbital-induced inhibition of CA secretion evoked by high K+ is accompanied by a decrease in Ca++ influx19). Generally, depolarization of the cells with elevated concentrations of K+ bypasses the ACh receptor and directly activates the voltage-dependent Ca++ channel. Lim and Hwang (1991)42) have reported that both DMPP and McN-A-343 greatly cause CA secretion from the isolated perfused rat adrenal glands by a calcium-dependent exocytotic mechanism.
However, in the present inventigation, pentobarbital depressed the secretory effect of CA evoked by Bay-K-8644, which is known to be a Ca++-channel activator and to cause positive inotropy and vasoconstriction in isolated tissues and intact animals29–30) and to enhance basal Ca++-uptake31) and CA release32). This finding that pentobarbital inhibited CA secretory responses by Bay-K-8644, as well as cholinergic receptor-stimulation and membrane depolarization, suggests that the inhibitory effect of pentobarbital is mediated through the blockade of Ca++ entry into the chromaffin cells.
In support of these ideas, there in now sizeable literature demonstrating a key role of Ca++ influx through voltage-sensitive Ca++ channels as a physiological pathway for activation of adrenal CA41, 43–47). Moreover, it is found that the activation of nicotinic receptors stimuates CA secretion by increasing Ca++ entry through receptor-linked and/or voltage-dependent Ca++ channels in both perfused rat adrenal glands48) and isolated bovine adrenal chromaffin cells20, 49–50), and that the muscarinic receptor activation causes an increase in adrenal CA secretion independent of extracellular Ca++ in various species51–53) and in cytosolic free Ca++ in boving isolated adrenal chromaffin cells without associated CA secretion47, 54–56). However, recently, Lim and Hwang (1991)42) have reported that removal of extracellular Ca++ depresses CA release evoked by DMPP or McN-A-343.
High K+-induced CA release from adrenal chromaffin cells is now known to consist of the following processes: depolarization of membrane, Ca++ influx through voltage-dependent Ca++ channels, elevation of intracellular Ca++ and activation of the machinery of CA release by the elevated intracellular Ca++57).
In the light of these previous findings, the present results that pentobarbital suppresses CA secretory responses induced by membrane-depolarization and cholinergic stimulation, as well as by Ca++-channel activator, strongly suggest that this inhibitory effect of pentobarbital may be exerted through the direct inhibition of voltage-dependent and/or receptor-linked Ca++ channels, resulting in blockade of Ca++ entry into the adrenomedullary chromaffin cells. This hypothesis is supported by the findings that the high K+-induced CA release from adrenal chromaffin cells is considered to be regulated by the increased intracellular Ca++20, 47).
Also, in the present study, pentobarbital did fail to affect CA secretory effect evoked by cyclopiazonic acid, which is known to be a highly selective inhibitor of Ca++-ATPase in skeletal muscle sarcoplasmic reticulum26–27) and a valuable pharmacological tool for investigating intracellular Ca++ mobilization and ionic currents regulated by intracellular Ca++28). These results illustrate that inhibitory effects of pentobarbital on CA release evoked by cholinergic stimulation and/or membrane-depolarization may be not associated with intracellular Ca++ mobilization. It is shown that Ca++-uptake into intracellular storage sites susceptible to caffeine58) was almost completely abolished by treatment with cyclopiazonic acid during the preceding Ca++ load40). This finding is consistent with the results revealed in skinned smooth muscle finbers of the longitudinal layer of the guinea-pig ileum, where Ca++-uptake was also inhibited by cyclopiazonic acid59). Suzuki and his coworkers (1992)28) have suggested that cyclopiazonic acid easily penetrates into the cytoplasm through the plasma membrane, reduces Ca++- ATPase activity in sarcoplasmic/endoplasmic reticulum, decreases the subsequent Ca++ release from those storage sites and, thereby, reduces Ca++-dependent K+-current.
Based upon these results, the present experimental data demonstrate strongly that the inhibitory effect of pentobarbital on the evoked CA release may be mediated, through direct inhibition of Ca++ influx into the chromaffin cells without intracellular Ca++ mobilization from those storage sites.