Acute Pyelonephritis Focusing on Perfusion Defects on Contrast Enhansed Computerized Tomography(CT) Scans and Its Clinical Outcome
Article information
Abstract
Objectives
Many cases of acute pyelonephritis show renal perfusion defects on contrast enhanced computerized tomography (CT) imaging studies. The purpose of this study is to show the frequency of renal perfusion defects in uncomplicated acute pyelonephritis and to compare the clinical responses of patients who had perfusion defects or not.
Methods
We studied patients who had symptoms and signs of acute pyelonephritis through CT examinations with contrast enhancement. We identified 21 cases who had perfusion defects among 35 patients who had undergone CT imaging studies and compared the clinical data in the two groups of patients who had perfusion defects on CT (group 1) and who had not (group 2).
Results
Nearly all patients had typical symptoms and signs of acute pyelonephritis such as high fever and chill, flank pain and costovertebral angle tenderness. Combined clinical problems were septic shock (one case, 4.8%) and disseminated intravascular coagulation (DIC) (one case, 4.8%) in group 1. Laboratory findings were not different between the two groups. All patients were treated with antibiotics and had successful recoveries. The duration of recovery of pyuria in group 1 (5.2±9.6 days) was not longer than that in group 2(3.1±2.9 days) (p>0.05). The length of defeverscence in group 1 (7.0±4.6 days) was longer than in group 2 (3.5±2.7 days) (p<0.05). There were no differences between group 1 and group 2 in the rate of predisposing factors. Thirteen of 21 cases (61.9%) in group 1 and five of 14 cases (35.7%) in group 2 had positive urine culture results which are relatively low probably due to the administration of antibiotics prior to our emergency room visit. Perfusion defects on CT were very frequent findings (60.0% of the clinical acute pyelonephritis patients). We classified CT findings of group 1 as focal unilateral (2 cases, 9.5%), multifocal unilateral (14 cases, 66.7%) and multifocal bilateral (5 cases, 23.8%), and there were no differences between the subgroups of group 1 in the duration of defeverscence.
Conclusion
Those patients who had perfusion defects on CT showed relatively severe clinical courses but responses to early antibiotics were very good. Contrast enhanced CT scans may be very sensitive for the detection of acute renal parenchymal inflammatory disease and for defining the extent of disease, but it is clinically not essential to perform in the early uncomplicated acute pyelonephritis because CT diagnosis does not change management. Clinical use of contrast enhanced CT scan may be appropriate in case of persistence of fever and leukocytosis for more than seven days despite antibiotic treatment.
INTRODUCTION
Acute renal infection was not an interesting field for the nephrologist despite the fact that it is the common form of all renal diseases. Acute pyelonephritis is a conventionally used clinical term in the case of upper urinary tract infection. The British Medical Research Council Bacteriuria Committee defined acute (bacterial) pyelonephritis as a clinical syndrome of loin pain, tenderness, and pyrexia accompanied by laboratory signs of bacterial infection of the kidney, including leukocytosis, pyuria, bacteriuria and a positive urine culture, sometimes with bacteremia and hematuria, as well1). Many other terms are also used in cases of upper urinary tract infection based on various imaging study findings in the literature, such as segmental acute pyelonephritis2), acute bacterial nephritis3), acute lobar nephronia4,5), acute focal bacterial nephritis4,6,7) and acute (diffuse and focal) pyelonephritis8,9). In the past decade, the development of CT scan and renal scintigraphy have elicited many interesting publications which showed new insight on a common disease. Talner et al recently published an outstanding review on ultrasound, scintigraphy and CT imaging of renal infection, focused on terminology10). The purpose of this study is to show the frequency of renal perfusion defects in uncomplicated acute pyelonephritis and to compare the clinical responses of the patients who had perfusion defects and who had not.
PATIENTS AND METHODS
We studied patients who had symptoms and signs of acute pyelonephritis during a 16-month period (from November 1994 to March 1996) and identified 21 cases of perfusion defects among 35 patients (60.0%) who had undergone contrast enhanced CT imaging studies in clinical acute pyelonephritis patients. Charts were reviewed to compare clinical responses of the patients who had perfusion defects on CT (group 1) and who had not (group 2). We analyzed CT findings and clinical characteristics of the patients to determine therapeutic responses and their clinical outcome. There were 19 females and 2 males, ranging in age from 18 to 73 years (mean±SD, 42.5±15.7 years) in group 1 and 13 females and 1 male, ranging in age from 16 to 55 years (mean±SD, 38.6±12.6 years) in group 2 (Table 1). CT examinations were performed on an GE 8800 (GE Medical systems, Milwaukee, USA) scanner with the use of 10mm section thickness. All patients had unenhanced scanning followed by intravenous contrast material-enhanced scanning.
STATISTICAL ANALYSIS
We used SPSS PC program for analyzing data. Chi-square test, unpaired t-test, and Kruskal-Wallis test were used to compare the data and P value lower than 0.05 were considered to be significant.
RESULTS
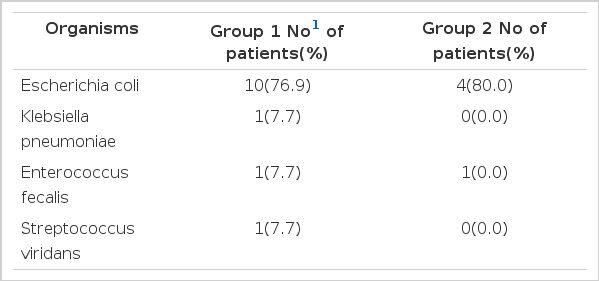
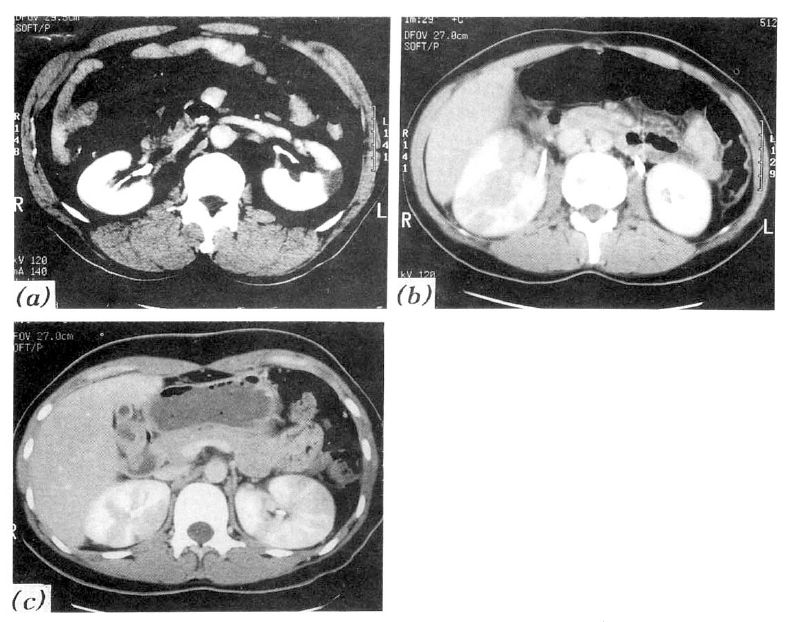
Nearly all patients had typical symptoms and signs of upper urinary tract infection such as high fever and chill, flank pain and costovertebral angle tenderness. Combined clinical problems were septic shock (one case, 4.8%) and DIC (one case, 4.8%) in group 1. Laboratory findings were as follows; pyuria 14 cases in group 1 (66.7%) and 10 cases in group 2 (71.4%), bacteriuria 6 cases in group 1 (28.6%) and 4 cases in group 2 (28.6%), microhematuria 11 cases in group 1 (52.4%) and 6 cases in group 2 (42.9%), leukocytosis 13 cases in group 1 (61.9%) and 7 cases in group 2 (50.0%) and thrombocytopenia 8 cases in group 1 (38.1%) and 2 cases in group 2 (14.3%). There were no differences between group 1 and group 2 in the rates of symptoms, signs, and various laboratory data (Table 1). All patients were treated with antibiotics (cephazolin + tobramycin) and had successful recoveries. The duration of recovery of pyuria in group 1 (5.2±9.6 days) was not longer than that in group 2 (3.1±2.9 days) (p>0.05). The length of defeverscence in group 1 (7.0 ±4.6 days) was longer than in group 2 (3.5±2.7 days)(p<0.05) (Table 1). In group 1, twelve of 21 cases (57.1%) had predisposing factors such as diabetes mellitus (3 cases, 14.3%), previous history of urinary tract infection (7 cases, 33.3%), recent history of obstetrical and gynecologic instrumentation (1 case, 4.8%) and chronic alcoholism (1 case, 4.8%) and, in group 2, six of 14 cases (42.9%) had predisposing factors such as diabetes mellitus (2 cases, 14.3%) and history of UTI (4 cases, 28.6%) (Table 2). There were no differences between group 1 and group 2 in the rate of predisposing factors (Table 2). Thirteen of 21 cases (61.9%) in group 1 and five of 14 cases (35.7%) in group 2 had positive urine culture results. Organisms cultured were as follows; Escherichia coli 10 cases in group 1 (76.9%) and 4 cases (80.0%) in group 2, Klebsiella pneumonia 1 case in group 1 (7.7%), Enterococcus faecalis 1 case in group 1 (7.7%), and Streptococcus viridans 1 case in group 1 (7.7%) and 1 case in group 2 (20.0%) (Table 3). Perfusion defects on contrast enhanced CT were very frequent findings (60.0% of the clinical acute pyelonephritis patients). We classified CT findings of group 1 patients as follows; focal unilateral (2 cases, 9.5%), multifocal unilateral (14 cases, 66.7%), and multifocal bilateral (5 cases, 23.8%) (Fig. 1) (Table 4). There were no differences between the subgroups of group 1 in the duration of defeverscence (Table 4).
DISCUSSION
Urinary tract infection (UTI) is one of the most common infections in female patients. Approximately 40% to 50% of women will have at least one UTI in their lifetime11). Ascending infection, probably due to reflux, is the most common route of infection, and hematogenous seeding is rare in acute pyelonephritis. Uncomplicated acute pyelonephritis usually responds well to appropriate antibiotics. Detection of renal abscess and other severe renal parenchymal infection is an important problem for clinicians who have to distinguish patients requiring more extensive medical therapy or surgical intervention from the vast majority of patients with uncomplicated acute pyelonephritis. Hill and Clark12) described the pathophysiology of acute pyelonephritis and its sequelae in the rabbit model. Profound cortical vasoconstriction was found in areas of acute inflammation with inflammatory cells clogging the peritubular capillary. After one week, these areas progressed to necrosis (abscess) and then to scarring. Contrast enhanced CT scan demonstrated various findings in the evolving stages of acute pyelonephritis. In the 1970’s, radiologists began to describe a subset of patients with acute renal infection who had very severe regional or generalized renal parenchymal abnormalities without apparent abscess, a severe protracted clinical course and eventual atrophy of the affected renal parenchyma3, 4). They use the term acute (focal) bacterial nephritis (acute lobar nephronia) in the subset of this category. The clinical feature of acute focal bacterial nephritis is similar to that of acute pyelonephritis and it is not possible to distinguish acute focal bacterial nephritis from acute pyelonephritis on clinical grounds. The spectrum of CT findings in acute renal infection depends on clinical severity. Huang et al. showed that renal bacterial infection may manifest the continuum of severity from uncomplicated acute pyelonephritis to acute bacterial nephritis and, finally, to frank abscess formation13). Talner et al10), in their outstanding review on terminology of acute pyelonephritis, suggested that the terms acute (focal) bacterial nephritis, acute lobar nephronia (nephritis), preabscess, renal cellulitis, renal phlegmon and renal carbuncle should not be used in orden to avoid confusion in terminology. In their review, patients with clinically mild and uncomplicated pyelonephritis appear normal on both pre- and post-contrast CT scan. With more severe infection, there is often renal enlargement or focal swelling with normal attenuation on precontrast scans. After contrast enhancement, findings are one or more wedge-shaped or streaky zones of low attenuation extending from papilla to kidney capsule. Zones of low attenuation on post contrast scans are almost certainly caused by focal ischemia, obstructed tubules and interstitial inflammation. Therefore, an accurate and precise description of the spectrum of imaging manifestations of this disease has now become important in order to ensure distinction from other focal renal diseases such as tumor, renal infarct and renal abscess8). We performed contrast enhanced CT examination on those who had symptoms and signs of clinically acute pyelonephritis at the time of visit to our emergency room to find out the frequency of focal inflammatory process and its clinical outcome. Perfusion defects on contrast enhanced CT scans are very frequently encountered in clinically uncomplicated acute pyelonephritis as shown in our cases. High fever, chill, flank pain, CVA tenderness and leukocytosis were almost universally present in our patients. Negative urine cultures present in 38.1% of group 1 and 64.3% of group 2 patients, which are very high in our cases, have been reported in up to 20% of patients with acute focal bacterial nephritis and renal abscess3–7,14). Low yield of urine cultures are probably due to the administration of antibiotics prior to our emergency room visit. The most common organism cultured is Escherichia coli which is present in about 80% of our cases and is similar to other reports11). Large proportions of our patients (57.1%) in group 1 had predisposing factors and these figures were similar to cited frequencies of up to 60% in previously reported series of patients with acute focal bacterial nephritis or renal abscess15). There were no differences between group 1 and group 2 in the rate of predisposing factors. Patients with perfusion defects (group 1) treated with antibiotics (cefazolin + tobramycin) became afebrile in about 7 days (mean duration of defeverscence, 7.0±4.6 days) which was longer than group 2. Clinical responses to antibiotics were very good and it was not necessary to use antibiotics for a prolonged period. Some patients had severe clinical courses such as septic shock and DIC which were resolved by early antibiotic treatment. Extent of perfusion defects, which was classified by CT pattern, did not influence the duration of defeverscence after admission. Pitfalls in CT diagnosis include failure to detect focal perfusion defects in studies performed without contrast enhancement and the overlap between the CT appearance of inflammatory, cystic and neoplastic disease in the kidney. As many as 5% of renal masses detected on CT scans are of indeterminate nature and require further evaluation16). There is disagreement in the literature about the best imaging modality for evaluating suspected renal infection17). Intravenous pyelogram is an insensitive test for acute pyelonephritis. Ultrasound examination is frequently chosen initially because of easy availability and relatively lower cost, but the majority of kidneys with uncomplicated acute pyelonephritis appear normal despite the frequent presence of perfusion defects on contrast enhanced CT scans10). In summary, those patients who had perfusion defects on contrast enhanced CT showed relatively severe clinical courses but responses to early antibiotics were very good. Contrast enhanced CT scans may be very sensitive for the detection of acute renal parenchymal inflammatory disease and for defining the extent of disease, but it is clinically not essential to perform in the early uncomplicated acute pyelonephritis because CT diagnosis does not change management. Clinical use of contrast enhanced CT scan may be appropriate in the case of persistence of fever and leukocytosis for more than seven days despite antibiotic treatment.