A Case of Carpal Tunnel Syndrome due to Dialysis-Related Amyloidosis in a Patient Undergoing Long-Term Hemodialysis
Article information
Abstract
Carpal tunnel syndrome (CTS) is characterized by burning pain, numbness and tingling sensation in the thumb, index and middle fingers and the lateral half of the palm and progressive atrophy of the thenar muscles by compression of the median nerve within the carpal tunnel due to a variety of etiologic factors. Surgical intervention usually successfully relieves symptoms of CTS. Recently CTS has been regarded as one of the major clinical manifestations of dialysis-related amyloidosis due to β2-microglobulin deposition and recognized with increasing frequency in patients undergoing long-term hemodialysis. We report a case of carpal tunnel syndrome due to dialysis-related amyloidosis in patients undergoing long-term hemodialysis, confirmed by electromyography and biopsy in transverse carpal ligament and median nerve.
INTRODUCTION
Carpal-tunnel syndrome (CTS) and destructive arthropathy associated with cystic bone lesions are the major clinical manifestations of dialysis-related amyloidosis in patients undergoing long-term dialysis treatment1). Dialysis-related amyloidosis has been recognized as a serious complication of dialysis therapy. Amyloid deposits were found in the carpal synovia and perineural tissues in long-term hemodialysis patients with CTS2). Previous study3) revealed that β2-microglobulin was the major constituent protein in dialysis-related amyloidosis. Carpal tunnel syndrome, which is one of the most frequent presentations of β2-microglobulin-associated amyloidosis, occurs with an increasing frequency in patients with end-stagerenal disease. Dialysis-related amyloidosis or β2-microglobulin-associated amyloidosis is noted much more commonly in older patients (older than 50 years), patients undergoing dialysis for more than 10 years, and those who have suffered from other rheumatologic diseases before developing renal failure4). After 10 years of dialysis, approximately 50% of patients surveyed exhibited the clinical manifestations of dialysis-related amyloidosis, and among patients who had survived with hemodialysis for 20 years, the prevalence of dialysis-related amyloidosis was reported to be almost 100%5).
Peripheral neuropathy is a common neurologic consequence of chronic uremia. As the symptoms and signs of peripheral neuropathy are similar to those of CTS, the diagnosis of CTS is often difficult and patients are sometimes misdiagnosed as having a peripheral neuropathy. Surgical management of the CTS usually provides the dramatic relief of the symptoms in most patients. However, to our best knowledge, carpal tunnel syndrome due to dialysis-related amyloidosis in patients undergoing long-term dialysis has not been reported yet in Korea. We hereby report a case of carpal tunnel syndrome due to dialysis-related amyloidosis in a patient undergoing long-term hemodialysis with a brief review of the literature.
CASE REPORT
A 55-year-old woman with end-stage renal failure due to chronic glomerulonephritis was hospitalized for severe pain, numbness and paresthesia in both wrists, with progressive paralysis of the thenar muscles and both shoulders’ pain for about 1 year. She had been on maintenance hemodialysis for 14 years at Chonnam University Hospital. Dialysis had been performed for 4 hours, 3 times weekly with hollow-fiber cuprophan dialyzers that were not reused. She had been on hemodialysis therapy since 1983 via a side to side cephalic vein-radial artery arteriovenous fistula in the left distal forearm and then, 5 years later, arteriovenous fistula in the right distal forearm due to loss of the function of the left arteriovenous fistula. Maintenance hemodialysis had been continued using this fistula. Symptoms mentioned above had been aggravated during hemodialysis and often awakened her at night.
On admission, blood pressure was 90/60mmHg, body temperature 37°C, pulse rate 94/min and respiration rate was 22/min. On physical examination, she was alert, obese and a chronically ill-looking appearance. She had pale conjunctiva and anicteric sclerae. Physical examination showed positive Phalen’s wrist flexion test and positive Tinel’s test at the wrist with thenar muscle atrophy. Her both knee joints were markedly swelled and their motions were moderately limited. On auscultation, her heart beat was regular and no murmur was heard. Her breathing sound was clear and no abnormal sound was heard. On abdominal palpation, liver, spleen or kidneys were not palpable. Peripheral lymph nodes were also not palpable. She had not suffered from tuberculosis or diabetes mellitus, but she had a trauma history at the right knee joint and the right thumb 4 years ago.
Laboratory findings were as follows: red blood cell count 2.87 × 106/mm3, white blood cell count 5.7 × 103/mm3, hematocrit 23.7%, hemoglobin 8.1 g/dL, platelet 179× 103/mm3 and the reticulocyte count 3.3%. Peripheral blood smear showed microcytic and hypochromic anemia (MCV: 80.3 fl, MCHC: 32.1%) with anisocytosis but preserved platelet and white blood cell counts. Blood chemistry studies showed serum electrolyte were normal, except for total serum calcium 8.1mg/dl and inorganic phosphate 6.6mg/dl. The AST was 65IU/I, ALT 51IU/I, alkaline phosphatase 143IU/I (liver origin 71.7%, and bone origin 28.3%), total bilirubin 0.4mg/dl, total serum protein 7.1g/dl and albumin 3.5g/dl, globulin 3.6g/dl and LDH 509IU/I. Renal function test revealed serum creatinine 8.6mg/dl, and blood urea nitrogen 44mg/dl and other laboratory tests showed that rheumatoid factor was 20IU/ml, C-reactive protein 2.8mg/dl, serum ferritin 210ng/ml, the erythrocyte sedimentation rate 78 mm/hr, serum β2-microglobulin 60.5ug/l (normal: 1.1 to 2.7mg/ml), intact parathyroid hormone 147pg/ml, 1α, 25(OH)2D3 8.9pg/ml (normal: 16 to 45pg/ml) and serum osteocalcin 13.9ng/ml (normal: 4 to 12ng/ml).
Radiologic examination showed bony destructive changes at right first interphalangeal joints in both hands and narrowing of joint space in right knee joint and bony destructive lesions with sclerotic border of left tibial plate, but otherwise nonspecific. Bone mineral density was examined, and the total body bone mineral density of our patient was within normal limit (1.191±0.0 g/cm2), compared with the normal controls (control group: 1.15± 0.10g/cm2). Whole body bone scan showed that hot spot uptake was noted in the areas of middle phalanx of right thumb, 4th and 5th left posterior ribs, left carpal bones, right distal femur and right lower sacroiliac joint.
Electrocardiography showed left ventricular hypertrophy, but otherwise nonspecific. Echocardiogram showed marked concentric hypertrophy with diastolic dysfunction.
Electromyographic examinations were performed in both median motor and sensory nerves: Nerve conduction studies demonstrated delayed distal latency and conduction velocity in both median motor nerves, and sensory nerves were not evoked and these findings were compatible with compression of the median nerves within the carpal tunnels (left side was more severely involved). Needle electromyography showed profusely abnormal spontaneous activities at rest and increased polyphasic Voluntary Motor Unit Action Potentials (MUAP) on minimal vibration in both abductor pollicis brevis innervated by median nerves.
On 7th hospital day, abdominal fat aspiration biopsy was done, and the biopsy was negative for amyloid on Congo red staining. On 15th hospital day, she underwent an open surgical release and epineurolysis at the left carpal tunnel with an impression of carpal tunnel syndrome. At the time of the surgical release and exploration of the carpal tunnel in left hand, she had moderate thickening of the transverse carpal ligament, hyperemia of the median nerve epineurium and compression and narrowing of the median nerve. Flexor tenosynovial hypertrophy on the left wrist and degeneration of transverse carpal ligament and synovium were seen, and dilatation of proximal fragment of left median nerve was also seen. Epineurolysis of left median nerve and left synovectomy were performed.
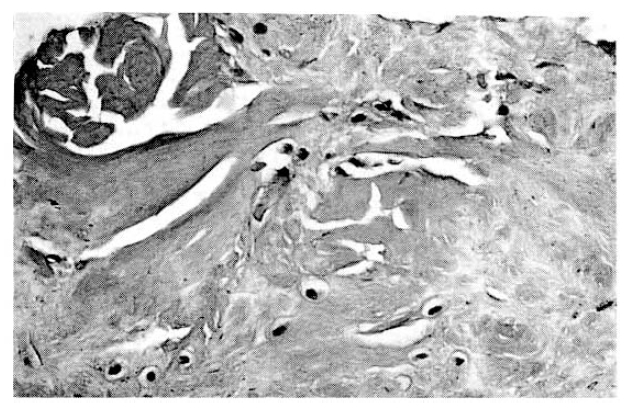
Histologic examinations of the transverse carpal ligament and epineurium of median nerve for amyloid deposition by Congo red staining, fluorescence microscopy and electronmicroscopy were performed. These tissues specimens showed multifocal amorphous pinkish deposits in the dense collagenous fibrous bundles in light microscopy, and red colored in hematoxylin and eosin, and Congo red staining (Fig. 1). The amyloid protein with Congo red staining exhibited apple-green birefringence under polarized light microscopy (Fig. 2) and electron microscopic examination showed numerous short but thick, nonbranching curvilinear fibrills aligned in parallel and aggregated in bundles (Fig. 3).
After surgical release of CTS, she had dramatic relief of pain and numbness in left hand and wrist joint during dialysis at night, did well for the following 3 months.
DISCUSSION
Carpal tunnel syndrome (CTS) as one of the major complications in patients undergoing long-term hemodialysis was first reported by Warren and Otieno in 19755). As the number of the patients and duration in hemodialysis increase, CTS has become an increasingly recognized problem in patients undergoing long-term hemodialysis. In a recent study6), the incidence of patients who required operation for CTS increased steeply from almost zero% before 8 years of maintenance hemodialysis to 50% at 14 years and almost 100% at 20 years.
CTS can start unilaterally but in many cases the contralateral joint soon becomes affected. Our patient had been on hemodialysis for 14 years and suffered from bilateral CTS. The etiology of CTS has not yet been firmly determined and several etiologic factors, including diabetes mellitus and rheumatoid arthritis, have been suggested7). Recently CTS has been reported to be associated with β2-microglobulin-associated and is now regarded as a major complication in patients undergoing maintenance hemodialysis4, 8). CTS and destructive arthropathy associated with cystic bone lesions are the major clinical manifestations of dialysis-related amyloidosis9).
Recent study10) shows that dialysis-related amyloidosis is of β2-microglobulin origin, and β2-microglobulin is an 11,800 dalton globular protein, and consists of 100 amino acids arranged in a single polypeptide chain. As the nonvariable light chain of the human class 1 major histocompatibility complex, β2-microglobulin is expressed on the surface of all nucleated cells. Gejyo et al.11) postulated that β2-microglobulin could not be removed efficiently from the blood by conventional hemodialysis and accumulated in tissues resulting in the formation of amyloid fibrils which, having a relatively high affinity to the carpal tunnel area, thus caused CTS. Although we did not directly examine the β2-microglobulin immunoreactivity in the amyloid deposit tissues, serum β2-microglobulin concentration markedly increased in the present case and this finding is compatible with the previous study12) that dialysis-related amyloid was of β2-microglobulin and conventional hemodialysis could not remove effectively the β2-microglobulin from the blood.
In CTS, amyloid fibrils are deposits in the carpal synovia, finger flexer tendon sheaths, transverse carpal ligaments and perineural tissues13). Histological finding was a pericollagenous distribution of the deposits associated with hyperplasia of the synovial tissue14). Our patient had very similar gross and histological findings when the carpal tunnel release operation as underwent and tissue specimens were obtained. On Congo red and hematoxylin and eosin staining, our patient’s specimens showed heavy deposition of Congo red positive materials that demonstrated green birefringence under polarized light microscopy, thereby characterizing the substance as amyloid. Electron microscopic examination in the present case showed the shorter and thicker nonbranching curvilinear fibirls aligned in parallel and aggregated in bundles, in contrast to the straight, longer and thinner fibrills seen in other types of amyloid, and these findings are compatible with a previous report15).
Radiologic examination in β2-microglobulin associated – amyloidosis demonstrates erosions and marginal defects of the affected bones, mainly at synovial insertion sites. A characteristic finding is periarticular cystic lesions, which grow in number and size with the continuity of renal replacement therapy. Those cystic bone radiolucencies arise from amyloid deposition in bone and thus are not ‘cyst’ in the true sense of the word. Commonly affected sites include the carpal bones, femoral and humoral heads, acetabulum, tibial plate and distal radius16). Radiologic examination in our patient showed destructive changes in many joints and bones, but typical cystic bony lesions were not noted.
Previous study17) reported that conventional hemodialysis using cuprophane membrane could not remove efficiently β2-microglobulin from the blood, but highly permeable biocompatible membrane including acrylonitrile, polysulfone or poly-anile membrane could more effectively remove and slow release of β2-microglobulin. Nomoto et al.18) recently reported that continous ambulatory peritoneal dialysis might minimize the emergence of CTS, although CTS was more likely to be one of the metabolic complications of end-stage renal failure itself. β2-microglobulin-associated amyloidosis is confined to those patients on nontransplant model of therapy4). Renal transplantation is the most effective method in preventing dialysis-related amyloidosis including CTS4, 19). So successful, early renal transplantation certainly prevents dialysis-related amyloidosis. Furthermore, in patients with already established dialysis-related amyloidosis, renal transplantation arrests the progression of radiologic signs of dialysis-related amyloidosis and produces an almost immediate abatement of osteoarticular origin20). Operative release of CTS gave a definite improvement of symptoms to almost all patients21) and after surgical intervention for CTS, our patient had also dramatic improvement in symptoms of CTS.
In conclusion, the carpal tunnel syndrome has been associated with dialysis-related amyloidosis due to β2-microglobulin deposition in patients undergoing long-term hemodialysis, and physicians should pay careful attention to the possibility of development in this major complication, especially in patients undergoing long-term hemodialysis.