Attenuated Central Pressor Response to Nitric Oxide Synthesis Inhibition in Chronic Renal Failure Rats
Article information
Abstract
Objectives
Central and peripheral roles of nitric oxide (NO) in blood pressure regulation have been suggested. The present study was aimed at examining if the role of NO in blood pressure regulation is altered in chronic renal failure.
Methods
Blood pressure responses to acute inhibition of NO were examined in 5/6 nephrectomized rats. Three weeks after the renal ablation, under thiopental (50 mg/kg, i.p.) anesthesia, an intracerebroventricuiar cannula was placed in the left lateral ventricle and the femoral vein was cannuiated to serve as an infusion route. The arterial blood pressure was measured in the right femoral artery. NG-nito-L-arginine methyl ester (L-NAME) was infused (100μg/kg per min for 60 min) either intracerebroventricularly or intravenously.
Results
Chronic renal failure rats showed a significantly higher arterial pressure than the control rats (147±14mmHg vs. 122±13mmHg). Intracerebroventricuiar L-NAME did not affect the arterial pressure in chronic renal failure rats (0.5±4mmHg increase from the basal), while it significantly increased the arterial pressure in normal rats (22±3mmHg increases from the basal). Intravenous L-NAME increased the arterial pressure, the magnitude of which did not differ between the normal and chronic renal failure rats (24±3 vs. 16±3mmHg increases from the basal).
Conclusion
These results indicate that the central role of NO in the regulation of blood pressure is altered in chronic renal failure.
INTRODUCTION
Among various vasoactive agents generated and released from the vascular endothelium, nitric oxide (NO) has been identified as one of the major relaxing factors, which is synthesized from the amino acid L-arginine by a family of enzymes, NO synthases1). These enzymes may be inhibited by L-arginine analogues such as NG-nitro-L-arginine methyl ester (L-NAME).
An inhibition of NO synthesis induces constriction of aortic rings isolated from various animal species, indicating that there is a continuous release of NO to maintain a dilator tone in the vasculature2). Furthermore, a single intravenous injection or continuous infusion of L-NAME causes a marked and sustained rise in blood pressure3,4).
Recent immunocytochemical studies have further detected varying amounts of NO synthase in most areas of the brain5,6). In addition to physiological functions of NO in memory7), vision8), feeding behavior9), nociception10) and olfaction11), a role in the central regulation of blood pressure was also demonstrated12,13).
On the other hand, the hypertension occurring in up to 80% of patients with chronic renal failure14,15) has been attributed to an accumulation of endogenous inhibitors of NO synthase, leading to impaired NO synthesis16). In addition, Kogosov et al.17) demonstrated in chronic renal failure rats an increase of norepinephrine contents after L-NAME treatment in brain nuclei involved in the neuroad-renergic control of blood pressure, which was normalized by L-arginine treatment. Although these findings suggest an altered NO physiology in chronic renal failure, to what extent it is affected has not been established.
The present study was aimed at examining if the role of endogenous NO in blood pressure regulation is altered in chronic renal failure. Arterial blood pressure responses to an acute inhibition of NO system were examined in 5/6 nephrectomized rats.
METHODS
Male Sprague-Dawley rats weighing 200–250g were used. They were maintained in accordance with Institutional Guidelines for Animal Care and Use. Surgical reduction of renal mass was carried out as previously described by other investigators18). Briefly, in rats under thiopental (50mg/kg, i.p.) anesthesia, the left kidney was 2/3 infarcted by ligating branches of the main left renal artery. The right kidney was tied off and removed. The rats were then allowed to recover, while they had free access to food and drink. Control rats were operated on in the same fashion except that the kidneys were manipulated without tissue destruction. Experiments were performed three weeks after the 5/6 nephrectomized or sham-operative procedures.
On the experimental day, the animals were anesthetized with thiopental (50mg/kg, i.p.). The right femoral artery was cannulated to measure arterial pressure. An intracerebroventricular cannula was placed in the left lateral ventricle and the femoral vein was cannulated to serve as an infusion route. A 30–60 min equilibration period was allowed to elapse until the protocol started. Basal data (arterial pressure) were obtained by averaging three values, recorded at least 5 min apart each, before the L-NAME infusion was started.
L-NAME (100μg/kg per min) was infused intracerebroventricularly at 1.25μL/min for 60 min. The same amount of L-NAME was infused intravenously at 40μL/min for 60 min. The control group was infused either intracerebroventricularly or intravenously with the vehicle, artificial cerebrospinal fluid.
L-NAME was purchased from Sigma Chemical Company. Data were expressed as means±SEM. Statistical significance was assessed using either nonpaired t-test or analysis of variance with repeated measures on time.
RESULTS
Three weeks after renal ablation, the rats showed (+++) or (++++) urine protein. Blood urea nitrogen and serum creatinine also significantly increased in chronic renal failure rats (Table 1). Basal arterial pressure was significantly higher in the chronic renal failure rats than in the control (Table 1).
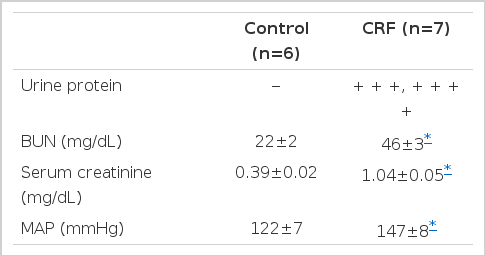
Comparison of Urine, Protein, Blood Urea Nitrogen (BUN), Serum Creatinine and Mean Arterial Pressure (MAP) between the Chronic Renal Failure (CRF) and Control Rats
Intracerebroventricular infusion of L-NAME (100μg/kg per min for 60 min) increased the arterial pressure in control rats (Fig. 1). However, it was without effect on the arterial pressure in chronic renal failure rats (Fig. 2).
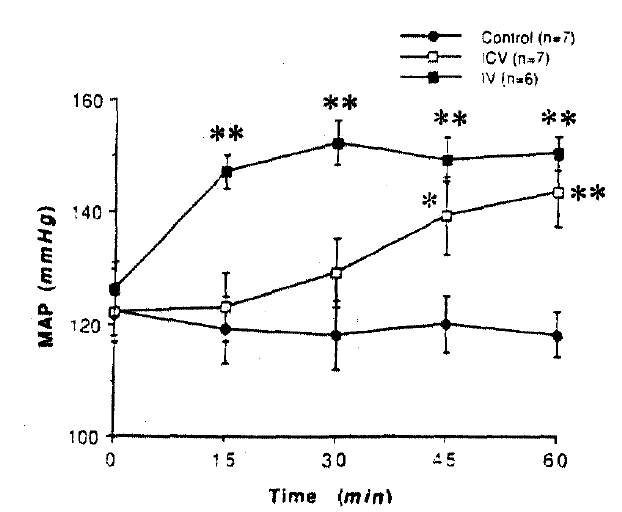
Mean arterial pressure (MAP) before and during intracerebroventricular (ICV) and intravenous (IV) infusion of L-NAME in normal rats. L-NAME was infused (100μg/kg per min) from 0 to 60 min. Control group was intracerebroventricularly infused with vehicle. n=number of animals. *: p<0.05, **: p<0.001; compared with 0 min value in each group.
Intravenous infusion of L-NAME (100 μg/kg per min for 60 min) increased the arterial pressure not only in control but in chronic renal failure rats (Fig. 1 & 2). In addition, the magnitude of maximal pressor response did not significantly differ between control and chronic renal failure rats(24± 3 vs. 16±3mmHg increases from the basal).
DISCUSSION
As has been previously observed13,19,20), L-NAME infused either intracerebroventricularly or intravenously increased the arterial pressure in normal rats, suggesting that endogenous generation of NO has a tonic inhibition on the blood pressure. The pressor response to L-NAME thus presumably reflects removal of the depressor or vasodilator action of the endogenous NO system.
The arterial pressure in chronic renal failure rats was, as expected, significantly higher than in control. An increased activity of the sympathetic nervous system has been implicated in hypertension in patients with end-stage renal disease21–23). The reversible sympathetic overactivity may be mediated by an afferent signal arising in the failing kidneys24).
On the other hand, an attenuated NO system has been proposed in chronic renal failure. Vallance et al.16) have suggested that an accumulation of endogenous asymmetrical dimethylarginine, leading to impaired NO synthesis, contributes to the development of hypertension associated with chronic renal failure. The accumulated endogenous inhibitors in chronic renal failure rats may result in an enhanced vascular tone. The speculation is further supported in chronic renal failure (5/6 nephrectomized) rats by the finding that L-arginine supplementation decreased the blood pressure17). Taken together, vasoconstriction may not only be indirectly enhanced by the overactive sympathetic activity but also directly by the reduced NO system in chronic renal failure.
In our study, intracerebroventricular infusion of L-NAME increased the arterial pressure in normal rats, but not in chronic renal failure rats. On the contrary, intravenous infusion of L-NAME caused pressor responses, the magnitude of which did not differ between the control and chronic renal failure rats. These findings suggest that the central NO mechanism is altered in chronic renal failure without changes in the peripheral mechanism. In support of our results, it has been shown that the norepinephrine content in the posterior hypothalamus and locus coeruleus was reduced by supplementation of L-arginine in chronic renal failure17).
Mechanisms underlying the discrepancy between the central and peripheral effects of L-NAME in chronic renal failure rats cannot be accounted for in the present study. However, we postulate that, in the presence of endogenous NO synthase inhibitors accumulated, basal sympathetic activity and vascular tone are higher than normal so that they may not be fully responsive to exogenously-administered NO synthesis inhibitors. Further studies will be needed to delineate the mechanisms for the altered responsiveness to the blockade of NO system.
