Complete Resolution of Airway Hyperresponsiveness in Aspirin-sensitive Asthmatic Patients
Article information
Abstract
Appreciable numbers of aspirin-sensitive asthmatic patients have chronic steroid-dependent severe asthmatic symptoms. We report four cases of aspirin-sensitive asthmatics who had mild to severe asthmatic symptoms, whose methacholine PC20 level ranged from 0.6 to 22 mg/ml at the first visit. The aspirin sensitivity was confirmed by lysine-aspirin bronchoprovocation. After anti-asthmatic medications and avoidance of salicylate-containing agents, airway hyperresponsiveness and respiratory symptoms disappeared for two to 30 months. These results suggest that early detection and careful avoidance of salicylate-containing agents may have a beneficial effect resulting in the resolution of airway hyperresponsiveness in aspirin-sensitive asthmatic patients.
INTRODUCTION
Aspirin (ASA) and non-steroid anti-inflammatory durgs (NSAIDs) can induce bronchoconstriction in 10% to 20% of adult asthmatic patients1,2). In asthmatics with rhinosinusitis, polyps, or both, the proportion is as high as 30% to 40% even without a history of ASA-induced respiratory reaction3,4). An appreciable number (50%) of all ASA-sensitive asthmatics have chronic severe, corticosteroid dependent asthma5). There have been few reports on the clinical course of aspirin-sensitive asthmatic patients. Here we report four cases, all of whom had mild to severe asthmatic symptoms, and who recovered completely for two to 30 months.
MATERIALS AND METHODS
Four asthmatic patients whose aspirin sensitivity was confirmed by a positive lysine aspirin (L-ASA) bronchoprovocation test (BPT) participated in the study.
Atopy was defined as showing more than 2+ skin prick test reactions to two or more common inhalant allergens such as Dermatophagoides farinae, alder, oak, rye grass, mugwort, ragweed and Aspergillus spp. (Bencard Allergy Service Brentford, Middlesex, U.K.). After their sensitivity to aspirin was confirmed, they were recommended to avoid salicylate-containing food and aspirin/NSAIDs, and treated with anti-asthmatic medications including inhaled and/or oral corticosteroid. No subject had had an upper respiratory tract infection or an exacerbation of asthma within the four weeks prior to the study.
1. Methacholine Bronchial challenge Test
Airway hyperresponsiveness to methacholine was determined by a method previously described6). An aerosol of 0.9% NaCl, followed by serial doubling concentrations of methacholine (0.075 to 25 mg/ml), was inhaled. FEV1 was measured 5 minutes after each inhalation until the FEV1 had fallen by 20% from the post-saline value. Airway hyperresponsiveness was considered to the present if a patient showed more than a 20% decrease in FEV1 after inhalation at any concentration (0.075 to 25 mg/ml) of methacholine. The PC20 value was obtained from the dose-response curve.
2. L-ASA Bronchoprovocation Test
L-ASA bronchoprovocation test was modified from a method described previously7). Pulmonary function was measured by a spirometer (Chest) and the FEV1 and maximum mid-expiratory flow (MMEF) before and during provocation. All medication, including theophylline, bronchodilators and steroids were stopped 24 hours before the test. The test solutions were delivered by a DeVilbiss 646 nebulizer (DeVilbiss Co. Somerset, Penn.) and connected to a compressed air-source (5l/min). Normal saline was inhaled as a placebo solution. The patients were asked to breathe the nebulized aerosol ten times up to their vital capacity. L-ASA (ASPEGIC®, Seoul, Korea), in powder form (1800 mg of L-ASA puls 200 mg of glycine) was dissolved in 10 ml of normal saline to produce an L-ASA solution of 180 mg/ml. The challenges with placebo were performed seven days before the L-ASA BPT. If there was less than a 10% fluctuation of FEV1 with placebo inhalation, L-ASA BPT from 11.25 up to 180 mg/ml was performed to determine the provocative dose of L-ASA which induced more than a 20% fall of FEV1. All provocations were carried out at the same time of day. The FEV1 and MMEF were measured every ten minutes during the first hour, and then hourly for the next 8 hours.
RESULT
Clinical characteristics and laboratory findings
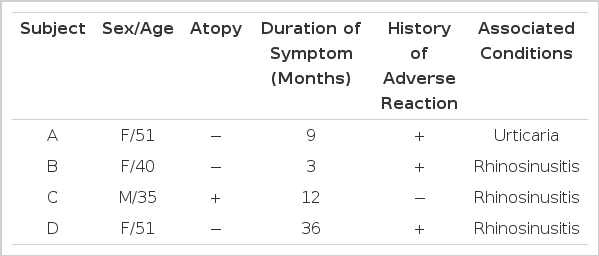
Three of the patients were non-atopic and the remaining one was atopic with no definitive causative allergen. Three had rhinosinusitis symptoms and the remaining one had urticaria. Three had a history of adverse reaction to salicylate-containing drugs or foods. Duration of asthmatic symptoms before diagnosis ranged from three to 36 months as shown in Table 1.
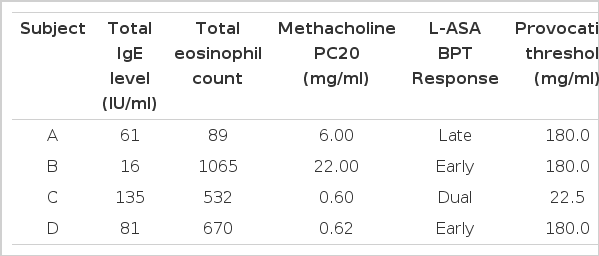
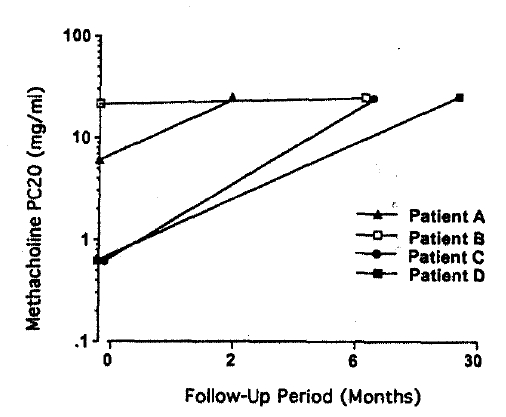
Table 2 shows laboratory findings at the first visit. Their blood eosinophil count ranged from 89 to 1065/mm3 and serum total IgE level measured by PRIST was not elevated. Methacholine PC20 level ranged from 0.6 to 22 mg/ml. Various types of asthmatic responses were elicited by lysine-aspirin bronchoprovocation test. In two to 30 months of follow-up, the patients’ asthmatic symptoms were much improved and airway hyperresponsiveness disappeared as shown in Fig. 1.
DISCUSSION
For adult asthmatics who reported a prior history of ASA-induced respiratory reaction, the prevalence of ASA sensitivity during oral ASA challenges ranged from 66% to 97%2,3,8). Our previous study9) revealed that 36.1% of intrinsic asthmatic patients showed positive responses on oral aspirin provocation. In the present study, all of them had intrinsic type of asthma, although one patient was sensitized to house dust mite.
The definitive diagnostic test for respiratory sensitivity has been oral provocation with ASA and NSAIDs. L-ASA inhalation has become an alternative diagnostic test to detect ASA sensitivity in asthmatic patients8,10–12). In our previous study7), inhalation of L-ASA induced late asthmatic responses as well as early reactions. In the present study, two patients had an early asthmatic response and the other two had a late onset asthmatic response (one dual and one isolated late onset response). These results, which we compared with changes in FEV1 following placebo inhalation, indicate that these were true late asthmatic reactions, and not diurnal variations.
The mechanisms of ASA-induced bronchoconstriction have been partly clarified. It is widely accepted that ASA and NSAIDs block the cyclooxygenase pathway, allowing the arachidonate substrates to be preferentially diverted down to the 5-lipoxygenase pathway. The product of 5-lipoxygenase can mediate chemotaxis (leukotriene B4) and are potent bronchoconstrictors and secretagogues13,14). The leakotriene-receptor antagonist may have a beneficial effect in aspirin-induced bronchoconstriction15).
Relevance of either an IgE or other possible immune mechanism is unlikely. There has been little work on identifying a specific IgE antibody to acetyl-salicylic acid16). Our previous study7) revealed that none of the dual asthmatic responders to the L-ASA bronchoprovocation test showed a positive skin prick reaction to L-ASA. Slader et al.17) observed the recruitment of mast cells, lymphocytes and eosinophils in BAL studies following L-ASA inhalation in aspirin-sensitive asthmatic patients. These cellular infiltrations might be involved in the development of aspirin-induced asthmatic responses.
Aspirin-sensitive asthmatic patients showed a wide clinical spectrum18). While 81% of them had severe steroid-dependent asthma, others had mild symptoms and two (8.7%) did not have airway hyperresponsiveness, although they showed significant bronchoconstriction on L-ASA bronchoprovocation test. The duration of asthmatic symptoms tended to be shorter in mild-degree asthma patients. In the present study, two had mild-degree airway hyperresponsiveness and the other two had severe-degree airway hyperresponsiveness, which diminished at the following methacholine bronchial challenge test, as did respiratory symptoms. The duration of asthmatic symptoms was less than 12 months, except in one case. Three patients had a history of adverse reaction.
These results suggest that early detection of aspirin-sensitivity through aspirin provocation test may be needed in patients who have non-atopic asthma with rhinosinusitis or skin symptoms, even though there is no history of adverse reaction. Cessation of exposure and treatment with anti-asthmatic medications, including corticosteroid, may induce a complete resolution of airway hyperresponsiveness in aspirin-sensitive asthmatic patients.