Changes of Atrial Natriuretic Peptide Level in Patients with Nephrotic Syndrome after Supine Bicycle Exercise
Article information
Abstract
Objectives:
The exact role of atrial natriutetic peptide (ANP) in the pathogenesis of edema in nephrotic syndrome (NS) has not been fully elucidated. We aimed to investigate the possible contribution of ANP to edema formation in NS.
Methods:
We subjected 18 nephrotic subjects and 20 healthy volunteers to supine bicycle exercise (SBE), a maneuver that seemed to increase venous return and to enhance the release of ANP. Plasma concentrations of immunoreactive-ANP were measured before and after SBE by radioimmunoassay.
Results:
There was a significant rise in the plasma concentration of ANP in the controls after SBE(from 31.1±6.16 to 42.0±6.01 pg/ml: p<0.05). Meanwhile, there was no change in plasma concentration of ANP in the patients with NS (from 35.4±6.04 to 35.1±5.31 pg/ml). The change in plasma concentration of ANP in controls was significantly different from that in those with NS (p<0.05). The mean baseline value of ANP in controls was the same as in NS.
Conclusions:
These results show that SBE was a simple maneuver to stimulate the release of ANP in healthy controls. In contrast, it failed to stimulate the release of ANP in subjects with NS.
INTRODUCTION
The mechanism by which nephrotic patients form edema has been a subject of controversy for many years. The traditional view is that the retention of sodium and water is a renal response to hypovolemia resulting from a shift of plasma water and electrolytes into the interstitial compartment because of diminished plasma oncotic pressure1). In recent years, however, evidence has been presented that some or even most patients with nephrotic syndrome have normal or increased blood volume during renal sodium and water retention2). These and other observations have suggested the possibility of intrarenal mechanisms for the sodium retention3).
Most recently the new hormone, atrial natriuretic peptide(ANP), has been discovered as possessing natriuretic, diuretic and vasodilating activities4,5). The hormone is synthesized, stored and secreted in the heart. The control mechanism of ANP releases from the atria has not been fully elucidated. Atrial distension or stretch has been reported as the most consistent stimulus for the release of ANP6,7). Recently, however, it has been suggested that the increased secretion of ANP in response to atrial distension is caused by the reduction of atrial distension and not by the distension itself8–10).
Because ANP likely plays a role in sodium balance and water homeostasis, we evaluated the possible involvement of ANP in the nephrotic syndrome, a condition in which extracellular fluid balance is abnormal3). To investigate the possible contribution of ANP to edema formation in nephrotic syndrome, we subjected nephrotic subjects and healthy volunteers to supine bicycle exercise without resistance, a maneuver that seemed to augment central blood volume, venous return and right atrial pressure physiologically and to enhance the release of ANP11).
METHODS
1. Study Population
Eighteen patients with nephrotic syndrome, who ranged in age from 15 to 45, comprised the experimental population. In each patient, urinary protein losses for 24 hours in excess of 3.5g were accompanied by hypoalbuminemia and edema. Renal biopsy revealed a variety of underlying glomerular diseases, including minimal change glomerulopathy (N = 5), focal and segmental glomerulosclerosis (N = 4), lupus associated proliferative glomerulonephritis (N = 3), Ig A nephropathy (N = 4) and hepatitis B virus-associated glomerulopathy (N = 2). Twenty healthy volunteers, aged 20 to 35, served as a control group. All denied a history of renal, hepatic and heart disease. They were found at the time of examination to be normotensive and to have a negative dipstick test for urinary albumin.
2. Study Protocols
Permission for the study was obtained from each patient and volunteer after a detailed description of the procedure and potential complications. Each subject remained supine on the bed equipped with a bicycle for at least 20 minutes before the exercise test in order to achieve hemodynamic stability. Immediately before the test, the baseline blood was sampled and systemic blood pressure was measured with a cuff and standard mercury sphygmomanometer and the radial pulse was checked for a minute. Then the subject exercised, supine on the bicycle, at 80 cycles per minute for 7 minutes. No complications occurred. Immediately after exercise, blood pressure and pulse were measured with a collection of blood samples. Blood samples for the measurement of ANP levels were placed immediately in ice-cold tubes containing 200 ul of a mixture of ethylenediamine tetraacetate(EDTA) (1 mg/ml of blood) and Aprotinin (1,000 KIU/ml). Samples for the measurement of renin activity were collected in ice-cold tubes containing EDTA. They were immediately centrifuged at 4 °C, 10,000g for 15 min, and the separated plasma was kept frozen at −20 °C until subjected to analysis.
3. Radioimmunoassay
Plasma immunoreactive ANP(ir ANP) was extracted using Sep-Pak C18 cartridges (Waters), as described by Cho et al.12). The recovery rate of the procedure estimated with 125I AP III added to the plasma was 70.5±1.2%. Radioimmunoassay(RIA) for ir ANP plasma levels was described previously12). The extracted samples were reconstituted with 100 ul of Tris-acetate buffer (0.1 M, ph 7.40, containing 0.2% neomycin, 10nM EDTA. 50 BAEE/ml SBTI, 0.02% sodium azide, 200 KIU/ml aprotinin and 1% bovine serum albumin). The addition of 100 ul of the anti-ANP antiserum was followed by 18–24 h incubation at 4 °C and then 100 ul of 125I-ANP were added. After 18–24 h incubation at 4 °C, the bound form was seperated from the free by charcoal suspension. Radioimmunoassay for plasma renin activity(PRA) was performed as reported previously by Cho et al.13).
4. Statistical Methods
Statistical significance was tested with Student’s t-test. All values are expressed as mean±SEM and the difference was considered significant at p<0.05.
RESULTS
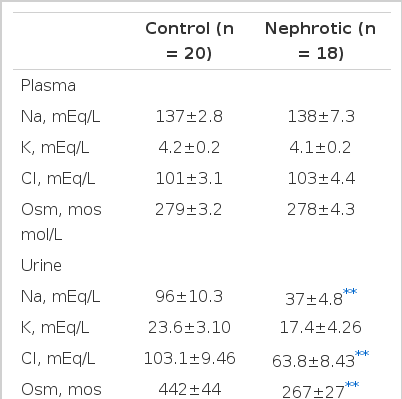
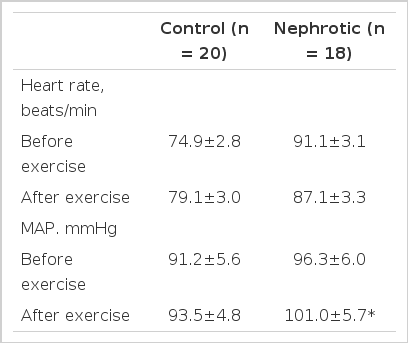
The plasma and urine measurements in controls and nephrotic subjects are summarized in Table 1. There was no difference in plasma levels of sodium or potassium. But urinary sodium, chloride and osmolality were lower in nephrotic patients than those of control subjects (p<0.01). As shown in Table 2, there was no significant change of heart rate and blood pressure in control subjects and no significant change of heart rate in nephrotics. But, mean arterial pressure in nephrotics increased during exercise (p<0.05). In spite of evidence of salt and water retention, there was no difference in the plasma levels of ir ANP between normal and nephrotic subjects (31.1±6.16 vs 35.4±6.04 pg/ml). Table 3 shows that controls demonstrated a significant increase in plasma ir ANP during the procedure(from 31.1±6.16 to 42.0±6.01 pg/ml ; p<0.05), while nephrotics did not show any significant change(from 35.4±6.04 to 35.1±5.31 pg/ml). PRA was a little higher in nephrotic subjects than in controls but there was no significant difference(3.23±0.28 vs 2.58±0.26 ng Al/ml/h). During the exercise, there was no significant change in PRA in controls and nephrotic subjects, as shown in Table 3.
DISCUSSION
ANP is known to have a significant role in water and elecrolyte balance and is known to play a counter-regulatory role in sodium retaining states like nephrotic syndrome. Studies of ANP levels in nephrotic patients have showed conflicting results. Some authors have reported elevated or depressed plasma levels of ANP in nephrotic patients14,15), while others were unable to document a clear-cut difference from controls16–18). In our study, in spite of sodium retention and the presence of edema, there was no significant difference in circulating ir ANP levels between nephrotic subjects and control subjects at rest as shown in Table 1, suggesting that sodium and water retention per se are necessarily associated with altered plasma levels of ANP10,16). The mechanism causing retention of sodium and water in the nephrotic syndrome are not fully elucidated. It has been proposed that the renin-angiotensin-aldosterone system, which is stimulated by hypovolemia, causes sodium and water retention19). But there was a lack of consistent reduction in blood and plasma volumes in patients with nephrotic syndrome. In one analysis, measured blood volume was reduced in only 30% and was normal or increased in the rest2). Micropuncture experiments in rats, in which nephrotic syndrome was induced by unilateral intrarenal injection of aminonulceoside, suggested that increase in tubular sodium reabsorption at the level of the collecting duct, rather than a decrease in the filtered load of sodium, is the primary mechanism of salt retention20). These results indicate that intrarenal mechanisms primarily account for the water and sodium retention in patients with nephrotic syndrome3).
The recent discovery of ANP and its likely role in natriuresis and diuresis raises the question of its involvement in the water and sodium retention of nephrotic syndrome. Two alternative possibilities are the blunted response of the nephrotic kidney to ANP or a defect in the atrial release of ANP in nephrotic syndrome21,22). The mechanisms by which ANP enhances renal excretion of sodium and water are not precisely known, but it is most likely a combination of an increased glomerular filtration rate and an inhibition of sodium reabsorption in the collecting ducts in the inner medulla23,24). The lack of renal response to ANP may be attributed to and intrarenal defect induced by the disease which causes the nephrotic syndrome14,25). Three intrarenal mechanisms for the blunted diuresis and natriuresis in response to ANP in nephrotic rats can be hypothesized22). First, in animal models of nephrotic syndrome, the blunted response to infused ANP is fully restored by prior renal denervation. This implies that it is augmented renal sympathetic activation, with consequent augmented proximal tubular reabsorption, which more than counteracts the effect of any increase in plasma ANP which may occur in these syndromes26). Second, ANP receptors in the kidneys of nephrotic rats may have an altered sensitivity to ANP. Perhaps the avid sodium retention in the nephrotic syndrome may decrease the density or affinity of renal ANP receptors, consequently decreasing the ability of ANP to induce diuresis and natriuresis15,27,28). Third, an interaction between ANP and angiotensin II may exist so that the natriuretic and diuretic effects of ANP are partially counteracted by the antinatriuretic and antidiuretic effects of angiotensin II22,29). Another important factor, which may modulate the action of ANP, is renal perfusion pressure. When renal perfusion pressure falls, the effects of infused ANP were diminished30).
The other possibility, a defect in the atrial release of ANP in nephrotic syndrome has prompted several studies to stimulate the release of ANP in nephrotic patients and rats15–17). These studies showed that nephrotic subjects have the ability to release ANP in response to volume stress by water immersion or albumin infusion. But, the maneuvers used were so strongly provocative that they caused a 20–36% expansion of plasma volume and a three to five-fold rise in the plasma level of ANP. So little is known about what will happen in nephrotic patients when they are exposed to minor stimuli to release ANP from the atria. In our study, supine bicycle exercise caused about a 35% rise in plasma levels of ANP without any significant increase in heart rate and blood pressure in control subjects. It is possible that nephrotic subjects have the capacity to release ANP at strong stimuli but fail to show a response to minor stimuli such as supine bicycle exercise. The reasons for the failure to release ANP in response to minor stimuli in nephrotic patients are not clear and the functional significance of a blunted release of ANP remains to be established. In addition, The possibility that nephrotic patients have less increment in venous return than controls cannot be excluded by this study. But it is unlikely that nephrotics have a decreased increment in venous return because most of them are normovolemic or hypervolemic2).
Although little is known about the release mechanisms of ANP, atrial distension6), changes in atrial pressure31), heart rate32) or atrial contraction frequecny7) are suggested as important stimuli for ANP secretion. The mechanisms by which atrial distension stimulates release of ANP have also yet to be defined. It is known that atrial distension causes a secretion of ANP by a direct local stretch effect rather than through atrial receptors6,7). More recently, it has been suggested that, the increase in secretion of ANP, in response to the atrial distension is caused by the reduction of atrial distension rather than by the distension per se8,9). The distension reduction-induced secretion of ANP may be caused by a myogenic control mechanism which is coupled with shortening of atrial muscle length10).
In the present study, we observed no significant suppression of PRA in control subjects in spite of an increase in ANP. This dissociation of PRA and ANP suggests that, although ANP may induced modulate renin secretion, other determinants(e.g., changes in renal-sympathetic nerve traffic) are likely to play a prominent role in regulating renin secretion following blood volume redistribution33).
Severe physical exercise is usually associated with increased blood pressure and heart rate and redistribution of systemic blood flow, leading to increased venous return and right atrial pressure34). So ANP increases significantly in response to exercise which increases right atrial pressure and heart rate, possibly mediated by the sympathetic nervous system11,35). Acute exercise stimulates ANP secretion in proportion to the intensity of exercise36). Our experimental protocols were designed to study the effects on circulating ANP levels of supine bicycle exercise known to increase central blood volume, venous return and mean right atrial pressure. As shown in Table 2, this maneuver did not increase heart rate and blood pressure in normal men which could change the circulating ANP levels36).
In summary, our present results show that supine bicycle exercise was a simple provocative maneuver to stimulate the release of ANP in healthy controls, while it failed to stimulate the release of ANP in patients with nephrotic syndrome. Our data suggest that there might be a blunted release of ANP in patients with nephrotic syndrome. Multiple factor might act in concert to produce edema in nephrotic patients and additional study will be necessary to characterize the role of attenuated ANP release and response in nephrotic syndrome and its pathophysiologic significance.
Acknowledgements
The authors thank Ms. Kyung Hwa Nam for excellent assistance and Ms. Yang Lim Jeoun for the preparation of the manuscript. This work was supported by a special research fund of 1995 from Chonbuk University Hospital.
Notes
This work was supported by a special research fund of 1995 from Chonbuk University Hospital.