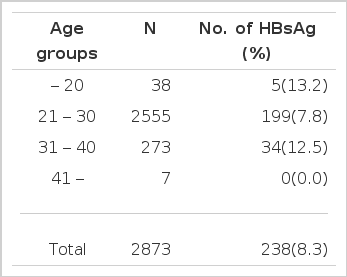Epidemiologic Study of Hepatitis B in Pregnant Korean Women
Article information
Abstract
The positive rates of hepatitis B viral markers according to many epidemiologic factors were analyzed in 2,873 pregnant women who delivered at St. Columban’s Hospital in Mokpo City from April 1st, 1985 to March 31st, 1986.
The following results were obtained:
The overall HBsAg positivity in all pregnant women was 8.3%.
The positive rate of HBsAg was unrelated to age. It was 13.2% in the 11–20 year age group. 12.5% in the 31–40 year age group, 7.8% in the 21–30 year age group and 0.0% in the 41–50 year age group.
The positive rate of HBsAg was slightly related to locality. It was a little higher in women who grew up in rural areas (8.6%) than in urban areas (7.7%).
The positive rate of HBsAg was unrelated to educational background.
The positive rate of HBsAg was unrelated to economic status. It was 8.7% in the highest income group and 8.6% in the lowest income group.
The positive rate of HBsAg was higher in cases who had injections more than four times.
The positive rate of HBsAg was higher, but not significantly, in cases who had received blood transfusion.
The positive rate of HBsAg was higher, but not significantly, in cases who had more than three siblings (0.05 <p <0.1).
There was no significant difference in HBsAg positivity if there had been a history of venereal disease.
The effect of a family history of liver disease: The positive rate of HBsAg showed significant increase if there was a family history of liver disease (p<0.005). The positive rates of Anti-HBs, Anti-HBc, HBeAg and Anti-HBe were increased, but not significantly, in cases with a family history of liver disease.
The effect of acupuncture. The positive rate of HBsAg showed a significant increase according to the freguency of acupuncture (0.025 <p <0.05). The positive rate of HBeAg was increased proportionately (0.05 <p <0.1), but the positive rates of Anti-HBs. Anti-HBc and Anti-HBe showed no difference according to the freguency of acupuncture.
The positive rate of Anti-HBs was significantly higher in student nurses (48.6%) and graduate nurses (58.0%) than in female university students (27.7%) (p<0.005). The positive rate of Anti-HBc tended to be higher (0.05 <p <0.1). The positive rates of HBsAg. Anti-HBs and Anti-HBc didn’t show any apparent tendency to increase in student and graduate nurses according to their year by year contact with patients.
INTRODUCTION
In 1967, after Krugman1,2) discovered that HBV infection is transmitted both parenterally and non-parenterally, many epidemiologic studies on Hepatitis B transmission routes were reported in Korea and in other countries.
Since HBsAg was detected not only in the blood but also in the saliva, tears, semen, vaginal secretions, menstrual blood, urine, pleural fluid, bile and breast milk,3–19) non-parenteral transmission of HBV is deemed quite possible. It would be attributed mainly to the environmental hygienic factors in this country.
The prevalence of HBV markers differ according to age, sex, locality, race, sexual behavior, socioeconomic, immunologic and genetic factors.20–24) Korea, located in the Far East is the most endemic area of HBV infection. The very high prevalence of HBV infection here might have been due to lack of hygienic facilities, limited family dwelling space and population density.22,25–27)
Although vertical transmission is deemed to be of importance in this country where the prevalence of HBeAg is very high, it will take a long time to discover the real mode of transmission of HBV infection here. Other than the many recent studies on vertical transmission28,30–34) and intra-familial infection,35,36) there are few reports about epidemiologic study on the environmental factors in HBV transmission.
In this study, to assess the possible influence of many environmental factors in the prevalence of HBV infection in Korea, we tested HBV markers in pregnant women, female university students, student nurses and graduate nurses who grew up in Mokpo City. The positivity of each viral marker was analyzed according to locality, educational background, income, frequency of acupuncture, family history of liver disease, frequency of injections, frequency of blood transfusions, number of siblings, history of venereal disease and the duration of contact with patients in the hospital.
MATERIALS AND METHODS
Two thousand, eight hundred and seventy three pregnant women who delivered at St. Columban’s Hospital in Mokpo, Jollanamdo, Korea from April 1st, 1985 to March 31st, 1986 were tested for HBsAg. Of these, 330 were selected for Anti-HBs, Anti-HBc, HBeAg and Anti-HBe analyses.
Forty seven Mokpo University Home Economics students, 72 Mokpo Holy Spirit College nursing students and 50 of St. Columban’s Hospital graduate nurses were selected for HBsAg, Anti-HBs and Anti-HBc analyses.
To observe the effect of contact with patients, we selected only those who had no history of liver disease in the family, who were not infected with clinical liver disease, who had no hepatitis vaccination, no acupuncture, who didn’t have many injections, who grew up in urban areas and whose economic status was middle class.
SGPT was determined by using MA 701 (IATRON Lab., Japan) and borderline cases were rechecked.
HBsAg, Anti-HBs, Anti-HBc, HBeAg and Anti-HBe were assayed by ELISA technique (Abbott Diagnostics. North Chicago, III). All sera were stored in a −20°C refrigerator and repeated when the result was doubtful.
RESULTS
1. Epidemiologic Factors and Positive Rates of HBsAg in 2,873 Pregnant Women
-
Positive rates of HBsAg according to age (Table 1)
Out of 2,873 pregnant women, 238 were positive for HBsAg, which is equivalent to 8.3%. The HBsAg positivity in the under twenties had the highest frequency, 13.2%. The 21–30 age group was 7.8% and the 31–40 age group was 12.5%. The over forties came out at 0.0%. There was no significant difference in HBsAg positivity according to age.
-
Positive rates of HBsAg according to locality (Table 2)
The survey was done on 2,873 pregnant women. Out of the 1,822 cases who grew up in rural areas, 157 or 8.6% showed HBsAg positivity. Eighty one or 7.7% of the 1,051 who grew up in urban areas were positive for HBsAg. The HBsAg positivity in pregnant women who grew up in rural areas was slightly higher.
-
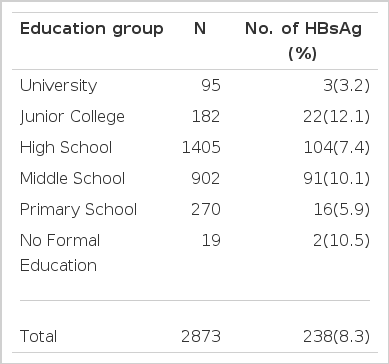
Positive rates of HBsAg according to ducational background (Table 3)
The survey was done on 2,873 pregnant women. The HBsAg positivity was 3.2% in female university graduates, 12.1% in female junior college graduates, 7.4% in female high school graduates, 10.1% in female middle school graduates, 5.9% in primary school graduates and 10.5% in women who had no formal education. There was no significant difference in HBsAg positivity according to educational background.
-
Positive rates of HBsAg according to economic status (Table 4)
We did a survey on 2,873 pregnant women divided into 5 income groups. The HBsAg positivity was 8.7% in the over 500,000 won income group, 7.8% in the 400,000–500,000 won income group, 9.0% in the 300,000–400,000 won income group, 7.8% in the 200,000–300,000 won income group, and 8.6% in the income group below 200,000 won. There was no significant difference in HBsAg positivity according to economic status.
-
Positive rates of HBsAg according to family history of liver disease (Table 5)
Our total survey consisted of 2,873 pregnant women. We eliminated 721 cases who had received acupuncture treatment more than once. Of the remaining 2,152 cases, 280 cases had a family history of liver disease and 1,872 cases didn’t. Thirty eight or 13.6% of 280 showed HBsAg positivity. One hundred and thirty one or 7.0% of 1,872 showed HBsAg positivity. The statistics indicate that the incidence of HBsAg positivity was significantly higher in cases with a family history of liver disease (p<0.005).
-
Positive rates of HBsAg according to frequency of acupuncture (Table 6)
Our total survey consisted of 2,873 pregnant women. We eliminated 317 cases with a family history of liver disease. We divided the remaining 2,556 cases into four groups. The HBsAg positivity was 6.7% in 1,953 cases who didn’t have acupuncture, 8.2% in 437 cases who had acupuncture 1–3 times, 7.8% in 102 cases who had acupuncture 4–10 times and 10.9% in 64 cases who had acupuncture more than 11 times. Statistics indicate that the incidence of HBsAg positivity was present more significantly in cases who had acupuncture more than 4 times (0.025< p<0.05).
-
Positive rates of HBsAg according to frequency of injections at the hospital (Table 7)
Our total survey consisted of 2,873 pregnant women. We eliminated 339 cases with a family history of liver disease and 66 cases who had received acupuncture treatment more than 11 times. Of the remaining 2,478 cases, the HBsAg positivity was 6.9% in 822 cases who received injections less than 3 times, 7.5% in 1,656 cases who received injections more than 11 times. Positive rates of HBsAg were increased according to the frequency of inactions but not significantly.
-
Positive rates of HBsAg according to frequency of blood transfusion (Table 8)
The survey was done on 2,873 pregnant women. The HBsAg positivity was 8.1% in 2,768 cases who had no blood transfusion, 14.5% in 55 cases who received 1–2 pints of blood, 10.0% in 30 cases who received 3–4 pints and 10.0% in 20 cases who received more than 5 pints. Positive rates of HBsAg were higher, but not significantly, in cases who had received blood transfusion.
-
Positive rates of HBsAg according to number of siblings (Table 9)
Our total survey consisted of 2,873 pregnant women. We eliminated 328 cases with a family history of liver disease and 66 cases who had received acupuncture treatment more than 11 times. Of the remaining 2,479 cases, the HBsAg positivity was 4.2% in 213 cases who had less than two siblings, but 7.6% in 2,266 cases who had more than 3 siblings. Positive rates of HBsAg were higher in cases who had more siblings, but not significantly (0.05 <p <0.1).
-
Positive rates of HBsAg according to history of venereal disease (Table 10)
The survey was done on 2,873 pregnant women. The HBsAg positivity was 8.3% in 2,812 cases who had no history of venereal disease and 8.2% in 61 cases who had. There was no significant difference in HBsAg positivity according to history of venereal disease.
2. The Positive Rates of HBV Markers in 330 Pregnant Women
-
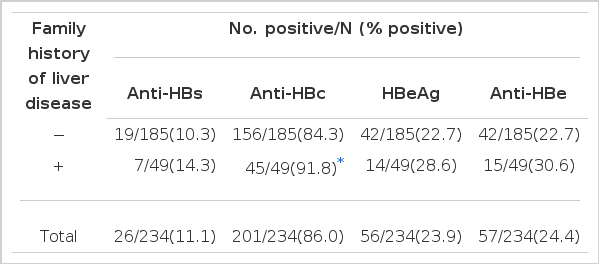
The effect of a family history of liver disease (Table 11)
We tested all 5 HBV markers in 330 pregnant women, of whom 243 were carriers and 87 non-carriers. Two hundred and thirty four of our 330 survey cases had no acupuncture treatment. Of 185 cases who had no history of liver disease, 38 or 13.6% were Anti-HBs positive, 156 or 84.3% were Anti-HBc positive, 42 or 22.7% were HBeAg positive and 42 or 22.7% were Anti-HBe positive. Among the 49 cases who had a family history of liver disease, 7 or 14.3% had Anti-HBs positivity, 45 or 91.8% had Anti-HBc positivity, 14 or 28.6% had HBeAg positivity and 15 or 30.6% had Anti-HBe positivity. The Anti-HBs, Anti-HBc, HBeAg and Anti-HBe positivities were higher in cases with a family history of liver disease.
-
The effect of acupuncture (Table 12)
We eliminated 69 who had family history of liver disease from our total survey of 330 cases. Out of 182 cases who had no acupuncture treatment, 21 or 11.5% were Anti-HBs positive, 154 or 84.6% were Anti-HBc positive, 42 or 23.1% were HBeAg positive and 40 or 22.0% were Anti-HBe positive. Of the 55 cases who had acupuncture treatment one to three times, 4 or 7.3% had Anti-HBs positivity, 44 or 80.0% had Anti-HBc positivity, 12 or 21.8% had HBeAg positivity and 14 or 25.5% had Anti-HBe positivity. Of the 16 cases who had acupuncture treatment four to ten times, 1 or 6.3% was Anti-HBs positive, 15 or 93.8% were Anti-HBc positive, 5 or 31.3% were HBeAg positive and 4 or 25.0% were Anti-HBe positive. Of the 8 cases who had acupuncture treatment more than eleven times, 1 or 12.5% had Anti-HBs positivity, 7 or 87.5% had Anti-HBc positivity, 4 or 50.0% had HBeAg positivity and 1 or 12.5% had Anti-HBe positivity.
The incidence of HBeAg positivity was progressively higher in cases who had a history of acupuncture treatment according to the frequency of acupuncture. There was no difference in the incidence of Anti-HBc and Anti-HBe positivities between those who had acupuncture treatment and those who had not.
-
Positive rates of HBsAg, Anti-HBs and Anti-HBc in university students, student nurses and graduate nurses according to their duration of exposure in hospital work (Table 13).
The Anti-HBs positivity was 27.7% or 13 of the 47 female university students, 48.6% or 35 of the 72 student nurses and 58.0% or 29 of the 50 graduate nurses. The statistics indicate that the Anti-HBs positivity was significantly higher in student nurses (0.01 < p<0.025) and in graduate nurses (p<0.005) than in female university students.
The Anti-HBc positivity was 47.0% or 22 of 47 female university students, 61.1% or 44 of 72 student nurses and 62.0% or 31 of 50 graduate nurses. The statistics indicate that the incidence of Anti-HBc positivity was higher in student nurses and in graduate nurses than in female university students (0.05<p <0.1).
Positive Rates of Anti-HBs, Anti-HBc, HBeAg and Anti-HBe According to Family History of Liver Disease
There was no significant difference between the three groups when observed for HBsAg positivity. But the number of cases surveyed in each group was small.
HBsAg, Anti-HBs and Anti-HBc positivities didn’t show any apparent tendency to increase in student and graduate nurses according to their year by year contact with patients.
DISCUSSION
High prevalence of HBV infection and many poor environmental conditions make epidemiologic study imperative in this country. The many studies reported on the prevalence of HBsAg were mostly observations of a specific hospital’s patients or a specific occupation’s employees within a short-term period.27,35–61) There are few reports of epidemiologic study on environmental and risk factors with long-term follow-up observation in relation to epidemiologic factors influential in the transmission of HBV.
In the course of our epidemiologic study to discover the real transmission mode of HBV in Korea including the perinatal, we made several interesting observations.
The prevalence in Korea of HBsAg according to age, sex, occupation and locality was reported by RPHA, double immunodiffusion, RIA and EIA methods.48–50) The HBsAg positivity of young pregnant women in our data was 8.3% by EIA and higher than 5.7% in women from Inchon by RIA in the study by Chang et al.40) But in the case of pregnant women in their twenties, our data revealed 7.8% and was slightly lower than 9.1% by Chang et al.40)
Szmuness23), Chang et al40) and Banke et al62) all reported that HBsAg positivity decreases with increase in age. But Ro et al45) found to the contrary that HBsAg positivity increases with increase in age. Our study revealed that HBsAg positivity in pregnant women showed no significant difference by age. It was same with Ahn et al’s report.43)
In foreign countries, the incidence of HBsAg positivity has been found to be higher in urban inhabitants than in people from rural areas.63,64) Ann et al43) reported that it showed no difference in the female according to locality. Our study showed that HBsAg positivity tended to be higher in people from rural areas (8.6%) than in those from urban areas (7.7%), but there was no significant statistical difference.
Although reports have shown that HBsAg positivity is lower in the well educated whose hygiene facilities and living conditions are good, our study found no significant difference according to educational background.
The HBsAg positivity was 8.7% in the highest income group and 8.6% in the lowest income group. There was no significant difference according to economic status.
It is a well known fact that Korea is one of the countries in the world with a high prevalence of HBV infection.27) The contaminated blood products brought in from abroad during the Korean War and the lack of hygiene facilities and the poor socioeconomic conditions prevailing after the War are believed to be the main causes.
At the time of World War II in 1942, over 50,000 U.S. Army personnel developed overt acute icteric hepatitis following yellow fever vaccination which was identified as contamination with HBV.29) Lack of sterile technique and facilities in rural health centers and in doctorless areas has also been one of the main contributing factors to the widespread HBV infection in Korea.
Krugman et al2) reported that 1cc of contaminated blood ingested was enough to get hepatitis B. Barker65) reported that 10,000-fold diluted blood containing HBV, given by intravenous injection, could cause hepatitis B.
Lack of sterile technique in acupuncture may also be an important factor in the transmission of HBV in Korea. Of the 2,873 pregnant women surveyed, 918 had received acupuncture treatment more than once. If we eliminated those 317 with a family history of liver disease, the HBsAg positivity would have been 8.5% in 601 cases. The HBsAg positivity was highest, 10.9%, in those who had acupuncture treatment more than eleven times. The HBsAg positivity was lowest, 6.7%, in those who didn’t have any acupuncture treatment (0.025 <p <0.05). According to the frequency of acupuncture, the positivity of HBsAg and HBeAg were progressively higher and the positivity of Anti-HBs and Anti-HBe were not changed. Acupuncture may be an influential factor in increasing the prevalence of both HBV infection and the carrier stage.
There have been many reports on HBV within residential and family dwellings. Blumberg et al71) and Carbonara et al72) reported that chronic HBsAg carrier can occur by autosomal recessive trait in accordance with Mendel’s law. Dumble et al73) reported the importance of the genetic factor, for example, the difference of HLA types and subtypes of HBsAg. Szmuness et al22,23) emphasized that besides the hereditary factor, the environmental factor has an influence.
Kim et al35) reported that HBsAg positivity in family members of 1,959 HBsAg positive blood donors was 29.4%. Lee et al36) reported that HBsAg positivity in family members of 74 patients with HBsAg positive liver disease was 26.7%. Kim et al44) reported that 12.5% of 869 HBsAg positive blood donors had a family history of liver disease.
Of the 2,873 pregnant women surveyed 1,001 had a family history of liver disease. If we eliminate the 721 cases who had acupuncture treatment more than once, the HBsAg positivity was 13.6% in the remaining 280 cases. The HBsAg positivity in cases with no family history of liver disease was 7.0% (p<0.005). In 330 cases with family history of liver disease, the positivity of Anti-HBs, Anti-HBc, HBeAg and Anti-HBe was increased but not significantly. The effect of acupuncture was slightly different with the influence of intra-familial infection.
Several foreign researchers reported that one source of HBV infection is the use of unsterilized needles when giving injections80,81). But this is not reported in Korea. In our study, the positive rate of HBsAg was 6.9% in 822 cases who hadn’t had injections or who had injections less than three times up to the time of delivery. But the positive rate of HBsAg was 7.5% in the 1,656 cases who had injections more than four times.
Some investigators have found that post-transfusion viral hepatitis B occurs as a result of blood transfusion82–84). But in Korea, Kim et al44) reported that a history of blood transfusion was noted only in 2.4% of 869 HBsAg positive blood donors. In our study, the positive rate of HBsAg was higher in cases who received blood transfusions (12.4%) than in those who didn’t (8.1%).
Jun et al61) reported that HBsAg positivity was higher the larger the family per room. In our study, the positive rate of HBsAg was higher, but not significantly, in cases who had many siblings.
There are a lot of reports about the transmission of hepatitis B by heterosexual or homosexual contact in foreign countries. But there are few reports in Korea and it would be difficult to do a study of this kind in Korea. Our study found no difference in HBsAg positivity according to the history of venereal disease. A study on sexual transmission of hepatitis B should be done on males in Korea with close observation and exclusion of other possible factors.
Many reports indicate that HBsAg positivity is high among hospital personnel and that these are more liable to become carriers.
Yu et al37), Mosley et al74) and Feldman et al76) found that among dentists and Lee et al46) reported that among doctors HBsAg positivity gets higher as they advance in their years of practice.
Hiroshi et al75) held that there was no change in HBsAg positivity but that Anti-HBs and Anti-HBc positivity were much higher in dental university students after one year of clinical practice.
Especially among graduate nurses, HBsAg positivity was found to vary in each reporter’s survey, as follows: Yu et al47). 6.3%, Yu et al37). 4.7% and Lee et al76). 5.4%. This variation is thought to be due to the sensitivity of test methods and to regional and environmental differences.
In our study, HBsAg positivity was 4.8% among 50 graduate nurses. Our result was a little lower because we observed cases free of other possible epidemiologic factors. Anti-HBs positivity was significantly higher in student and graduate nurses than in female university students (p<0.005) and the Anti-HBc positivity tended to be higher (0.05< p<0.1). This means that the more exposure there is to HBV, the greater is the incidence of HBV infection.
But the positivity of HBsAg, Anti-HBs and Anti-HBc showed no tendency to get higher as the duration of contact with the patients got longer. This finding might be due to the method of selection and to limiting it to small numbers.
Taking all the results into consideration, locality, sex, economic status and educational background can have an indirect bearing on the incidence of HBV infection, but acupuncture and intra-familial infection can have a more direct and significant influence. It has been seen that according to the frequency of acupuncture, it may be influential in increasing the prevalence of HBV infection and in decreasing antibody formation.
Because teenagers and those in their twenties have already shown a high prevalence of HBV infection, we must study many other environmental and hygienic factors with a view to improving them. We must also engage in epidemiologic study that is forward looking so that the young generation of the present and of the future will grow up in improved living conditions and receive HB vaccination.