Frequency of Detectable HBsAg in Fluid Adherent to the Endoscope, Gastric Juice, and Saliva Collected during Endoscopy in Patients Positive for HBsAg
Article information
Abstract
Gastric juice, saliva, and fluid adherent to the endoscope were collected from 50 patients who were seropositive for hepatitis B surface antigen (HBsAg) during the endoscopic examination of the upper gastrointestinal tract, and examined for HBsAg, using the radioimmunoassay. A positive test was obtained from 42.0% of the saliva samples, in 32.0% of the gastric juice specimens, and in 31.3% of the fluid adherent to the scope. These results should be taken as a warning, that calls for a more careful screening of the patients and disinfection of the endoscope.
INTRODUCTION
Upper gastrointestinal endoscopy with a flexible fiberoptic endoscope is a useful procedure that has been used for many years, but it involves the potential hazard of transmitting infectious agents, such as the hepatitis B (HB) virus. Glouberman1) and Whalen2) have suggested that transmission of the HB antigen by a fiberoptic endoscope is a distinct possibility, but it has not yet been proved conclusively. HB surface antigen (HBsAg) is detectable in all kinds of human secreta, and transmission of HB via the nonparenteral routes such as saliva, gastric juice, urine, and stool has been documented.3–5)
The dentist,6) surgeon,7) physician in oncology8) and physician in a dialysis unit9) have a higher incidences of serum HBsAg positivity than the physicians in other specialties. This suggests that frequent exposure to HBsAg is an important transmission route.
MATERIALS AND METHODS
Fifty patients, all seropositive for HBsAg as determined by the RPHA (reverse passive hemagglutination assay) method, underwent an upper gastrointestinal examination with a flexible fiberoptic endoscope. The diagnoses of these patients were viral hepatitis 19(38.0%), gastritis 14(28.0%), liver cirrhosis 11 (22.0), gastric ulcer 4(8.0%), and hepatocellular carcinoma 2 (4.0%) (Table 1).
As preparation for endoscopy, patients fasted from the evening before the examination day until the endoscopic procedure. Fifteen to thirty min, before the procedure, 1.0mg of atropine were injected intramuscularly. Pharyngeal anesthesia was give with 2% xylocain jelly before the examination. Olympus GIF-D, K type endoscopes were used for examination.
We collected fluid adherent to the endoscope (gastric juice, and saliva), by wiping it directly into small glass tubes. All of the samples were tested for HBsAg by the RIA (radioimmunoassay). In RIA, we used an Austria II-123 kit of Abbott Laboratories, Chicago, asssay procedure B. The cut off value is the net CpM of the negative control mean (NCx) times the factor 2.1.
RESULTS
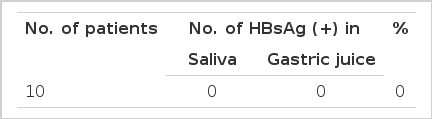
Gastric juice aspirated through the endoscope in the case of 50 patients was positive for HBsAg in 16 of the cases (32.0%) (Table 2). The saliva was positive for HBsAg in 21 (42.0%) (Table 3). The secretions adherent to the endoscope after extubation were positive in 15 of the 48 cases in which examinations were done (31.3%) (Table 4). As a control, 10 patients who were negative for HBsAg were similarly studied. The gastric juice, saliva and fluid adherent to the endoscope sampled during the endoscopic examination were all negative for HBsAg in ail 10 cases (Table 5).
DISCUSSION
Many investigators have suggested the possibility that HB is sometimes transmitted through endoscopy, but evidence of this has been lacking. We examined saliva and gastric juice collected during endoscopic examinations done on 50 carriers of HB, and material adherent to the endoscope. It has been shown that HBsAg positive serum is infective at a dilution of 1:107 if given parenterally,10) and it has been reported that 0.5ml of positive serum could transmit HB by an oral route,11) but the exact oral dose capable of transmitting the disease is not known.
There is epidemiological evidence that there are a large number of HBsAg particles in saliva, sufficient to transmit HB infection.5) Those reports suggested that even a minimal amount of HBsAg in saliva, gastric juice, and blood can effect transmission. In our study the test for HBsAg was positive in 42.0% of the saliva samples, in 32.0% of the gastric juice specimens, and in 31.3% of the material adherent to the endoscope.
Morris et al.12) performed endoscopy in 32 patients inadvertently following an emergency endoscopy in a patient who was later found to be positive for HBsAg. They followed these patients and tound that none of them developed jaundice or biochemical abnormalities. McDonald and Silverstein13) also reported that no clinical and biochemical signs of liver disease developed in 4 patients who underwent endoscopy with a scope that had been used in patients seropositive for HBsAg. A similar observation was reported by Axon et al.14)
Morris et al.12) and McDonald and Silverstein13) washed the endoscope thoroughly to remove adherent materials and cleaned it with 70% alcohol for each endoscopic examination, and suggested that the possibility of HB transmission was reduced to a minimum by this procedure. By contrast, there are many pessimistic physicians who doubt whether this disinfection procedure will absolutely prevent transmission of hepatitis by endoscopy.12–15) Although there was no incidence of endoscope-transmitted HB, Morris et al.12) began using 2% glutaraldehyde as a disinfectant. McDonald and Silverstein13) tried not to do endoscopy on patients who were positive for HBsAg and jaundiced. Similarly, Axon et at11) suggested that transmission of hepatitis by endoscopy is possible. Bond and Moncada15) recommend 2% glutaraldehyde or 8% formalin, because alcohol will not inactivate HB virus completely. According to Glouberman,1) 2% glutaraldehyde cannot destroy the infective particles, but the blood treated with 2% glutaraldehyde would not transmit virus even though HBsAg should still be present in the disinfected blood, because there is no relation between the viral infectivity and its immunologic activity that will give a positive test.16) He also suggested that HBsAg could be destroyed by exposing it to ethylene oxide gas for 16 hours. Besides ethylene oxide gas, 90°C moist heat for 2 hours. 85°C moist heat for 1 hour or 100°C for 10min, can destroy the infectivity. These method cannot be applied to fiberoptic instruments, because the maximum heat tolerance of the Olympus or ACMI fiberoptics is 50–60°C.
Of ethylene oxide gas and 2% glutaraldehyde. Axon et al.14) prefer the latter and suggest 15 min, of soaking in it because the former is time-consuming and more expensive.
In the endoscopy room of our hospital, we use the following procedure for disinfection : the fiberoptic endoscopes are washed thoroughly in running water, and 70% alcohol is used to cleanse them after the adherent materials have been removed.
Villarejos et al.17) and Almeida et al.18) emphasized that HB virus can be transmitted by air, and that the medical personnel exposed to the secreta of patients may become infected in a small endoscopic room. It has been reported that the bacteria in the oral cavity of a patient is demonstrable in the air,19) and some endoscopists suggest that both the endoscopist and his/her co-workers should wear masks to protect themselves.2) Chalmers et al.20) suggested that HBsAg-positive physicians, nurses, dentists, and other medical personnel can transmit HB virus to patients, based on an outbreak of hepatitis transmitted by such individual.21) Sultz et al.22) also noted that HB virus has been transmitted to patients through the wounded hands of an HBsAg-positive physician in the giving of injections, the manipulation of medical devices, or the intubation of an endoscope.
Although we did not investigate endoscopic biopsy forceps and cytology brushes, if contaminated by blood in the stomach of the patient positive for HBsAg, they could be a vehicle for transmission of HB virus infection. Bond et al.15) proposed that endoscopic accessories should be either disposable or exposed the required length of time to ethylene gas after each use, and Whalen2) suggested that a temperature of 250°C at 15 1bs, pressure for 15 min, would provide an adequate protection against HB virus.
In summary, the need for ensuring that the endoscope is free of contamination with HB virus is a real one, particularly in view of the fact that endoscopic cholangiopancreatography is now commonly used in patients with jaundice.