A Study on Cerebral Embolism in Mitral Stenosis
Article information
Abstract
To evaluate the significance of episodes of cerebral embolism in patients with mitral valve disease in Korea, 128 patients with echocardiographic diagnosis of mitral valve disease were examined. Among these, 82 patients had predominant mitral stenosis.
The clinical features of 82 patients with mitral stenosis have been reviewed to elucidate the factors favoring cerebral embolism which occurred in 19 patients, i.e., incidence of 23.2%.
Atrial fibrillation was present in 16 of 19 patients with cerebral embolism (84.2%). Cerebral embolic episodes occurred in 16 of 47 patients with atrial fibrillation (34.0%).
The mean age (55.3 ± 12.1 years) of patients without cerebral embolism was significantly older than that (43.2 ± 14.6 years) of patients without cerebral embolism (P<0.005).
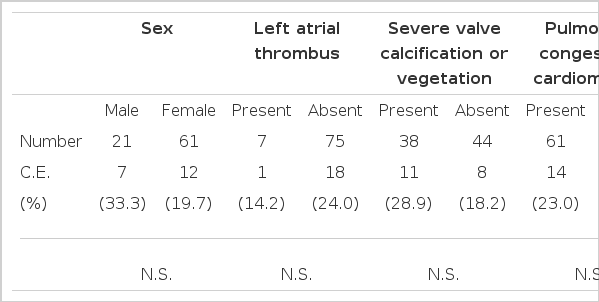
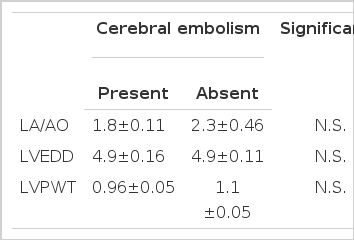
There was no significant relationship between the incidence of embolism and sex, left atrial thrombi, left atrium/aortic root diameter, mitral valvular orifice area, mitral valvular vegetation or calcification, left ventricular enddiastolic dimension or left ventricular posterior wall thickness.
Cerebral embolism is common in patients with mitral stenosis in our country. The presence of atrial fibrillation and low cardiac output increase the attack of cerebral emboli whereas the severity of mitral stenosis, as judged by valve area, may not correlate with the occurrence of emboli. The best treatment for cerebral embolism is prevention. Therefore, we believe that more vigorous treatment of patients with mitral valve disease who are old or associated with atrial fibrillation as well as previous embolic history is indicated.
INTRODUCTION
Cerebral embolism may have its thromboembolic source in the heart of the patients with various heart diseases such as rheumatic valvular heart diseases, myocardial infarction and dilated cardiomyopathy. Once the cerebrovascular accidents have occurred from the cerebral embolism, about 50 to 70% of the patients died or suffered from major morbidity immediately1).
Though the need to identify and prevent the patients at risk for cerebral embolism is obvious, there are few documented systematic approaches to answer the fundamental questions about it in patients with valvular heart diseases in Korea.
We reviewed 128 patients with mitral valvular diseases to analyze the significance of cerebral embolic episodes in mitral valvular diseases and to elucidate the risk factors.
SUBJECTS AND METHODS
1. Subjects
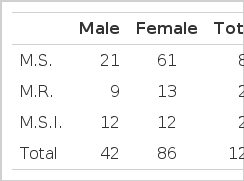
One hundred and twenty-eight patients were diagnosed as having mitral valvular diseases in Kyung Hee University Hospital by echocardiography from February, 1980 to August, 1982. Among these, 82 patients (male 21, female 61) had predominant mitral stenosis (Table 1).
2. Methods
Mitral stenosis was defined as a narrowed mitral valvular orifice area shown by two dimensional echocardiography.
The diagnosis of cerebral embolism was made when a patient had a cerebrovascular accident of sudden onset in the presence of mitral stenosis with the brain CT findings of infarction.
RESULTS
1. Sex and age
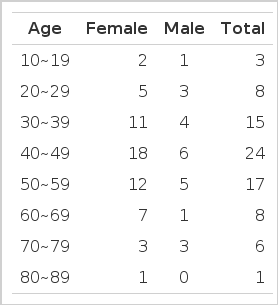
Women outnumbered men with the ratio of 2.9:1. The age ranged from 13 to 82 years (mean: 46 years). The problem was most frequently observed in thirties, forties, and fifties (Table 2).
Nineteen cerebral embolic episodes were complicated in the 82 patients with mitral stenosis (Table 3). The age of these patients ranged from 27 to 74 years (mean: 55 years). The problem was more frequently observed in the older age group with the highest incidence in forties, fifties, and sixties.
Atrial fibrillation was noted frequently in the forties, fifties and sixties, the mean being 51 years, somewhat lower than the mean age of cerebral embolism.
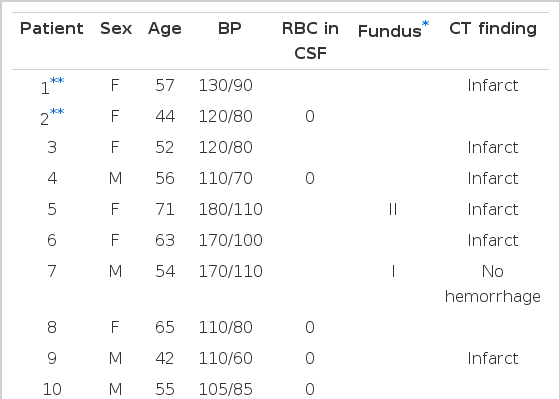
2. Characterization of the patients with cerebral embolism (Table 4)
Nineteen subjects with mitral stenosis were complicated by cerebral embolism. They were older than 40 years except one (patient 12) and were associated with atrial fibrillation except three cases (patients 2, 13, 15)
Blood pressure was within normal limits in 13 subjects among 19 patients. Six hypertensive patients showed cerebral infarction without any evidence of cerebral hemorrhage in brain CT, and had atrial fibrillation in all cases except one.
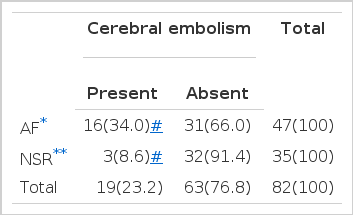
3. Atrial fibrillation in relation to cerebral embolism (Table 5)
While cerebral embolism developed in 16 patients of the 47 patients (34%) with atrial fibrillation, only 3 patients of 35 patients (8.6%) with normal sinus rhythm had an attack of cerebral embolism.
While atrial fibrillation was noted in 16 cases (84.2%) among 19 patients with mitral stenosis complicated by cerebral embolism, 31 cases out of 63 patients (49.2%) with mitral stenosis with no cerebral embolism had atrial fibrillation.
4. Left atrial thrombus, mitral valve calcification, pulmonary congestion, and cardiomegaly in relation to cerebral embolism (Table 6)
Left atrial thrombi were found in 7 patients (8.5%) of 82 patients with mitral stenosis. Among these 7 patients, one (14.2%) cerebral embolic episode developed. No statistically significant difference was observed in the occurrence of cerebral embolism between the patients with left atrial thrombi and those without. Severe mitral valve calcification, pulmonary congestion, and cardiomegaly did not significantly influence the occurrence of cerebral embolism.
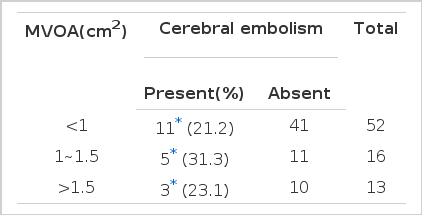
5. Mitral vavular orifice area by echocardiography in relation to cerebral embolism (Table 7)
Eleven (21.2%) cerebral embolic events occurred among the 52 patients whose mitral valvular orifice area(MVOA) was less than 1 cm2, 5 (31.3%) cerebral embolism occurred among 15 patients whose MVOA was between 1, and 1.5 cm2, and 3 (23.1 %) cerebral embolism occurred among 13 patients whose MVOA was over 1.5 cm2. Thus, no significant difference was observed between the groups with different size of MVOA.
6. Echocardiographic findings in relation to cerebral embolism (Table 8)
Left atrial dimension, left ventricular enddiastolic dimension, left ventriculr posterior wall thickness were analyzed in relation to cerebral embolism. No significant differences were found.
DISCUSSION
Patients with rheumatic heart disease and arteriosclerotic heart disease constitute the overwhelming majority of adults with cerebral emboli arising from the heart.1) Left atrial myxoma,2) mitral valve prolapse,3) cardiomyopathy,4) and atrial fibrillation5) make up a small group. Cerebral embolization during heart surgery including artificial valve replacement also call attention.6,7) Most patients with rheumatic heart disease have mitral stenosis and some also have mitral insufficiency and aortic valve disease.
Cerebral embolism consist of 5% of all cerebrovascular accidents in Korea,43,44) but how much mitral valve disease contributes to it is unknown. Congestive heart failure and systemic embolization have been known as the main fatal complications of mitral stenosis. Systemic emboli occur in 9–49% (average 10–20%) of patients with rheumatic heart disease and 20–70% (average 40%) of the systemic emboli are cerebral.8–12)
Despite the limitation of the small number of subjects during the short term period of our study, 19 cerebral embolic episodes were observed among the 82 patients with mitral stenosis, i.e., incidence of 23.2%, which reflected the same trend as in the previous reports. Mitral stenosis is prevalent in women (Male to female ratio; 1:2.5). However, the patients with cerebral embolism as a complication of mitral stenosis does not show any significant sexual difference in their occurrence (male to female ratio; 1:1.8).11,12)
In age distribution, mitral stenosis frequently manifests itself between thirty and forty years of age of the affected patients but cerebral embolism complicate it 5–10 years later, i.e., in their forties to fifties.12–15)
In our data of 32 patients with mitral stenosis, the male to female ratio was 1:2.9 and the prevalent age was thirties, forties, and fifties. The 19 complicated cases with cerebral embolism showed a male to female ratio 1:1.7 with prevalent ages of forties, fifties and sixties. Atrial fibrillation is a very important risk factor of cerebral embolization in the patients with mitral stenosis. 42–82% of rheumatic hearts are fibrillating or obviously changing rhythm at the time of embolization.14–17) A fibrillating heart has 3–6 times greater chance of releasing emboli than one in normal sinus rhythm.16–20) Philips reported that the longer the duration of atrial fibrillation, the greater the complication of the cerebral embolism.
Our data revealed that atrial fibrillation was noted in 16 of 19 patients with cerebral embolism (84.2%) and also showed that 16 (34%) of 47 patients with atrial fibrillation were complicated with cerebral embolism which indicates four fold higher incidence compared to the 3 (8.6%) occurrence among 35 patients with normal sinus rhythm.
However, the atrial fibrillation is not the sine qua non for the embolization. Confirmative diagnosis of cerebral embolism is not always possible; especially, the incidence of cerebral embolism in rheumatic heart disease with normal sinus rhythm is unclear.1,20)
Atrial fibrillation must be an important factor in cerebral embolization. The majority of mitral stenosis, however, are in normal sinus rhythm. Therefore, the risk of cerebral embolism should not be overlooked in the cases without atrial fibrillation.
Left atrial thrombi were observed in 5–20% of mitral stenosis during operation.9,21–23) Although the embolic risk has been well known during or after the operation in such patients, direct relation of preoperative embolization to left atrial thrombi is not clear.23–24) This is probably due to difficulty of preoperative diagnosis24) and uncertainty of preoperative history,23) that is, minor cerebral embolic episodes are difficult to be detected clinically. Recently two dimensional echocardiography enables us to manage the cerebrovascular accident patients better by virtue of its recognition of intracardiac tumor, thrombus, vegetation, and abnormalities in wall motion.25–27)
We reviewed the 7 cases with left atrial thrombus observed in two dimensional echocardiography in the light of cerebral embolization. Only one case (8.5%) of them was complicated with cerebral embolism. When compared with the 18 occurrences (24%) among 75 mitral stenosis patients without discernible left atrial thrombi, there was no significant difference between the two groups. Maybe, as Spangler has noted,27) the fact that atrial thrombi are smaller than ventricular thrombi, mostly located in left atrial appendage, technically difficult to be detected in the case attached to the left atrial posterior wall, fresh soft thrombi may be more difficult to be detected by echocardiography, and minor cerebral embolic history can hardly be recognized can be explanations to the above results. Of course, it is also noted that embolization immediately after the formation of thrombi can probably result in the rarity of left atrial thrombi.27)
We also studied severe valve calcification or vegetation, pulmonary congestion, cardiomegaly, mitral valvular orifice area, the dimension and the function of left atrium and left ventricle in these patients by echocardiography and compared them in the view of cerebral embolization but found no statistically significant differences between them.
Casella,11) Oleson,28) Graham,29) Traill,30) etc. had reported the similiar results. They suggested that the presence of atrial fibrillation and reduction in cardiac output increase the risk of cerebral emboli wherease mitral valvular orifice area (severity of mitral stenosis), left atrial dimension, cardiomegaly, intracardiac pressure, pulmonary congestion, New York Heart Association functional classification, sexual difference may not affect significantly the risk of cerebral emboli. Severity of mitral valve calcification has been described as having a significant relation with postoperative embolization only.11)
The immediate mortality from cerebral embolism is about 25–35% and immediate morbidity is approximately the same, thus approximately 50–70% of patients die or suffer major morbodity immediately.8,16,17,31,32) Furthermore it is shocking that tragic results may develop in the asymptomatic patients with mitral stenosis. Though it is often stated that one-half to two-thirds of all patients with cerebral embolism will die within one year, largely because of their serious primary cardiac disease, this estimate is not well documented. Wells17) noted that advanced age, prodromal symptoms, loss of consciousness and/or seizures at onset, and failure to improve within 48 hours, were the factors correlated with a “poor outcome.”
Once the cerebral embolism occurs, the risk of recurrence becomes higher. The natural recurrence rate of embolization is 30–75% in the patients followed for varying periods of time.8,11,17,18,32,33) The recurrences of cerebral embolism are frequently observed early in the follow up. One-fourth to one-third of recurrences occur within the first 2–4 weeks and one half to two-thirds of them occur within the first year.33,35) High probability of early recurrence in cerebral embolic patients with mitral stenosis leads us to acknowledge that its prevention is the best management.
Anticoagulant therapy should be started immediately if the patients with mitral stenosis are associated with cerebral embolism or have great risks for it. The incidence and the recurrence rate of cerebral embolism could be suppressed to 10–20% by anticoagulant therapy.33–36) The prevention of embolism by an operative method needs further evaluation. Many insisted that valvulotomy would reduce the embolic rate.9,28,37,39) But, many contradictory reports have been published.8–10) Mitral valve replacement has not been considered as a preventive method of cerebral embolism yet. It takes for granted that anticoagulant therapy should be employed after any operation.
All patients with prominent mitral stenosis are at risk for systemic embolism and should be anticoagulated with warfarin indefinitely.1,33–37) Whether the benefit of longterm anticoagulation outweighs the risk in patients with lesser degrees of mitral stenosis is unclear. The best treatment for cerebral embolism is prevention and, therefore, we believe that more vigorous management of patients with thrombus-prone mitral stenosis is indicated. This includes patients with obvious mitral stenosis who are old or associated with atrial fibrillation as well as previous embolic history.