A Case of Transition of Polycythemia Vera to Chronic Neutrophilic Leukemia
Article information
Abstract
Chronic Neutrophilic Leukemia (CNL) is a rare myeloproliferative disorder characterized by a persistent increase of mature peripheral neutrophils, myeloid hyperplasia in bone marrow, hepatosplenomegaly, elevated neutrophil alkaline phosphatase (NAP) and absence of Philadelphia chromosome, with no evidence of infection or malignancy sufficient to mimic a leukemoid reaction. CNL has been associated with multiple myelomas in many reported cases, but transition of Polycythemia Vera (PV) to CNL is very rare.
An 81-year-old female patient, who had undergone intermittent phlebotomy following the diagnosis of PV 8 years previously, was admitted to our hospital due to lower back pain. A physical examination showed a splenomegaly 2cm below the costal margin, with tenderness of the thoracic and lumbar spine area. A peripheral blood examination showed a WBC count of 91,800/μL (neutrophil 88%) with a rare immature form, hemoglobin of 9.1 g/dL and a platelet count of 1,661,000/μL. Her NAP score was 58. The bone marrow examination showed 95% cellularity, with an M:E ratio of 10:1, increased megakaryocytes with normal morphology and the absence of myelofibrosis. Chromosomal studies showed no Philadelphia chromosome. A radiological examination showed compression fractures of the vertebrae and spinal cord compression. No underlying disease causing a leukemoid reaction was detected. With iron replacement, the hemoglobin level failed to increase over 12 g/dL. Therefore, it was concluded to be a transition of PV to CNL. After administration of hydroxyurea and vertebroplasty, the symptom improved and the WBC count was sustained below 40,000/μL.
INTRODUCTION
Polycythemia vera (PV), idiopathic myelofibrosis, essential thrombocythemia (ET), chronic myeloid leukemia and rarely chronic neutrophilic leukemia (CNL) are classified as chronic myeloproliferative disorders, with their pathophysiologies involving clonal expansion of a multipotent hematopoietic progenitor cell and the overproduction of one or more of the formed elements of the blood. These entities may transform into acute leukemia or be transmitted to each other. Of these, PV is a prototype of chronic myeloproliferative disorders, and often terminates in myelofibrosis or acute leukemia. The transition of PV to other types of chronic myeloproliferative disorder, especially CNL, is very uncommon.
CNL is a rare myeloproliferative disorder characterized by a persistent increase of mature peripheral neutrophils, myeloid hyperplasia in bone marrow, hepatosplenomegaly, elevated neutrophil alkaline phosphatase (NAP) and the absence of Philadelphia (Ph) chromosome, with no evidence of infection or malignancy sufficient to mimic a leukemoid reaction1). While the nature of CNL remains to be fully elucidated, there is enough evidence to support its myeloproliferative nature2–5).
Herein, the case of an 81-year-old female patient with CNL, which transformed from PV 8 years following its diagnosis, is reported. To the best of our knowledge, this is the first case report of the transition of PV to CNL in South Korea.
CASE REPORT
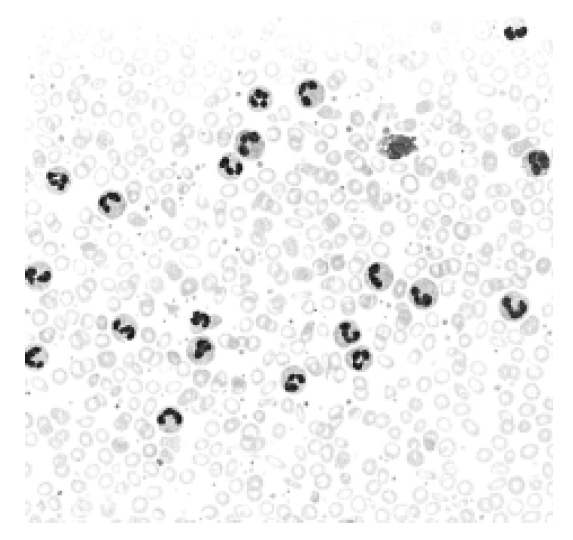
An 81-year-old woman was admitted to Hallym University Sacred Heart Hospital in May 2003 due to lower back pain. From her previous history, she had been treated with phlebotomy on several occasions after the diagnosis of PV in Australia during April 1995. At that time, her WBC count was 21,400/μL (neutrophil 92%), hemoglobin 20.3 g/dL, and platelet count 290,000/μL, the SaO2 was 96%, NAP score 108 and vitamin B12 909 pg/mL. A physical examination showed a splenomegaly 2 cm below the costal margin, with tenderness in the thoracic and lumbar spine area. Her WBC count was 91,800/μL (myelocyte 1%, metamyelocyte 1%, band neutrophils 2%, neutrophil 88%, lymphocyte 2%, monocyte 3%), hemoglobin 9.1 g/dL (MCV 63.5 fL) and platelet count 1,661,000/μL. A peripheral blood smear showed microcytic hypochromic anemia, neutrophilic leukocytosis and thrombocytosis (Figure 1). Her iron, TIBC and ferritin were 10 μg/dL (normal range 50–170), 353 μg/dL (normal range 250–450) and 11.43 ng/mL (normal range 13–150), respectively. Her LDH level, NAP score and VB12 were 566 IU/L (normal range 260–460), 58 (normal range 30–130) and 1873 pg/mL (normal range 225–1,100), respectively. Erythropoietin was 10.1 MIU/mL (normal range 10.2–25.2) and SaO2 93.3%. Monoclonal gammopathy was not detected by protein electrophoresis. Bone marrow aspiration and a biopsy showed 95% cellularity, with a myeloid:erythroid (M:E) ratio of 10:1, markedly increased granulocytic precursors with normal maturation pattern and 2% myeloblast and increased megakaryocytes with normal morphology, but no evidence of myelofibrosis, and a decreased stainable iron level (Figure 2, 3). Chromosome studies of marrow cells revealed a normal karyotype, no Ph chromosome and no bcr-abl rearrangement. A spine MRI showed compression fractures of the T11, L1,2,4 and leukemic infiltration of the cervical, thoracic, lumbar spine (Figure 4). No underlying disease causing a leukemoid reaction was detected. There was no other cause of the iron deficiency anemia, with the exception of previous phlebotomy and a rectal ulcer on sigmoidoscopy. To rule out persistent PV, iron was administered. However, the hemoglobin level failed to increase over 12 g/dL. Based on these findings, the patient was judged to have a disease that had been transformed from PV to CNL. After the administration of hydroxyurea, at the conventional dosage, and vertebroplasty, her symptom improved and the WBC count decreased to 38,900/μL (neutrophil 92.8%, lymphocyte 4.9%, monocyte 1.6%), hemoglobin 10.7 g/dL with MCV 81.5 fL, and a platelet count 470,000/μL. She did not revisit our hospital after July 2003, and died in October 2003 (information from the record of national health insurance corporation).
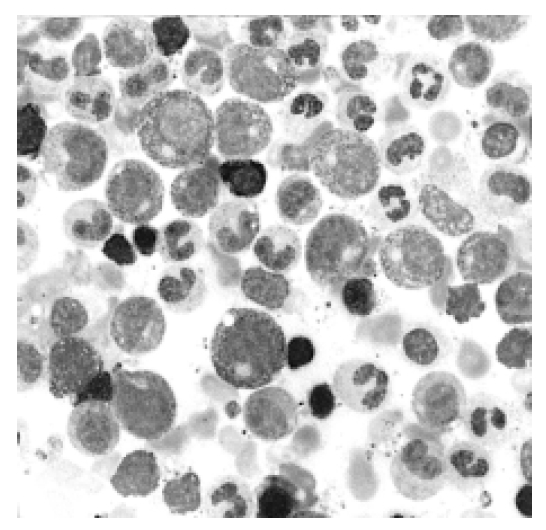
Bone marrow aspiration smear shows marked granuloytic hyperplasia, with a normal maturation pattern (Wright stain, ×400).

Bone marrow biopsy is hypercellular for her age. Granulocytic precursors are markedly increased. Megakaryocytic precursors are also increased (H & E stain, ×200).

(A) T1-weighted image, (B) T2-weighted image, (C) enhanced T1-weighted image with fat suppression view
Spine MRI findings are:
diffuse signal change, with mild enhancement of the vertebral body, from the lower thoracic to the lumbar spine.
multiple compression fractures.
posterior protrusion of the T11 body, with spinal cord compression -> these findings are suggestive of leukemic infiltration.
DISCUSSION
In 1920, Tuohy6) described the first recorded case of an unusual sustained neutrophilia, with splenomegaly, but without fever, inflammation, cancer or other cause of a leukemoid reaction. The term ‘chronic neutrophilic leukemia’ was first used by Tanzer et al7). in 1964, while Jackson & Clarke8) reported a case of ‘neutrophilic leukemia’ a year later. These early cases, however, as well as many subsequent reports, often lack sufficient data to support a definite diagnosis. With more than 100 cases of CNL having been reported since that time, a clear understanding of the disease’s pathogenesis, natural history and prognosis is warranted9). Despite these limitations, there are well characterized cases that meet the criteria for CNL, which cytogenetic or molecular genetic studies have confirmed clonality of the neutrophil lineage2–5). The Steering Committee for the WHO classification of Neoplastic Disease has recently acknowledged CNL to be a distinct myeloproliferative disorder, with the recommendation all other causes mimicking CNL should be excluded10).
There are some cases of CNL in association with multiple myeloma, but only a few cases of transition of PV to CNL have been described11–14). From the data of the 6 previously reported cases, the ages at diagnosis of PV were between 39 and 63 years old, and all patients were men. From the time of diagnosis of PV to the transition to CNL ranged from 5 to 17 years, with the transition being accompanied by a decrease in the hemoglobin level in all instances (from 17.8–21.6 to 10.1–12.7 g/dL). The number of cases is too small to assess the influence of the administered agents during the PV phase upon the process of transition, but all cases received myelosuppressive chemotherapeutic agents, such as P32, chlorambucil, busulfan, pipobroman, ranimustine, 6-mercaptopurine, carboquone and hydroxyurea. Various chemotherapeutic agents, such as hydroxyurea, 6-thioguanine, 6-mercaptopurine, busulfan, low dose cytarabine and cyclophosphamide, were tried, but the accumulation of further clinical experience is obviously still needed for the proper management of patients with transition to CNL.
In our case, the time from diagnosis of PV to the transition to CNL was 8 years, and the transition was accompanied by a decrease in the hemoglobin level, as in the other reports (from 20.3 g/dL to 9.1 g/dL). She received intermittent phlebotomies on several occasions during the PV phase, but used no chemotherapeutic agents, including hydroxyurea. She had iron deficiency anemia, and there was no other cause other than the previous phlebotomies and a rectal ulcer on sigmoidoscopy. After iron replacement, to rule out persistent PV, her hemoglobin level failed to increase over 12 g/dL. Unlike other reports, our case showed marked thrombocytosis, to 1,661,000/μL, after transition. A peripheral blood smear did not show either platelet aggregation or giant platelets. BM studies showed no abnormal morphology of megakaryocyte suggestive of ET, only increased numbers of megakaryocytes. Thus, it was presumed that our patients had undergone a transition from PV to CNL. Because hydroxyurea was not used during the PV phase, it was started as a treatment of CNL, and was relatively effective in controlling the leukocytosis, which reduced the WBC count from 91,800 to 38,900/μL.
Despite the unclear understanding of CNL, there have been some CNL cases confirming the clonality of the neutrophil lineage2–5). Therefore, the case described herein, along the six others previously reported cases were PV transformed to CNL, indicate that derangement at the hematopoietic stem cell level may produce characteristics of CNL. These support the contention that CNL may indeed represent a myeloproliferative disorder arising from stem cells.
Although 14 cases of CNL have been reported in South Korea since 198715, 16), the transition of PV to CNL has not. Therefore, herein, the case of an 81-year-old female patient, with the transition of PV to CNL 8 years after the diagnosis, is reported.