High dose Chemotherapy and Autologous Stem Cell Transplantation for Poor Risk and Recurrent Non-Hodgkin’s Lymphoma: A Single-Center Experience of 50 Patients
Article information
Abstract
Background
The long-term survival of patients with non-Hodgkin’s lymphoma after conventional chemotherapy is about 35%, with the remaining 65% of patients tending to be refractory or experience relapse. As such, primary refractory patients responding to salvage chemotherapy, and sensitive relapsed patients and primary high-risk patients are recommended to receive high-dose chemotherapy (HDC) and autologous peripheral blood stem cell transplantation (PBSCT). We evaluated the role of HDC and autologous PBSCT in patients with primary refractory, primary high risk, and sensitive relapsed non-Hodgkin’s lymphoma.
Methods
We performed a retrospective analysis of the data from 50 patients with non-Hodgkin’s lymphoma who were treated with HDC and autologous PBSCT in the Catholic Hematopoietic Stem Cell Transplantation Center between 1997 and 2002.
Results
Of the 50 patients, the conditioning regimen was BEAM in 20, CMT (cyclophosphamide, melphalan and thiotepa) in 19, fludarabine- and total body irradiation (TBI)-based regimen in 8, and cyclophosphamide and TBI in 2. There were 3 (6%) deaths due to treatment-related toxicity within the first 50 days after transplantation. Twenty-five patients remain alive at a median follow-up duration of 40.5 months (range 9–61). Among the patients with partial response before transplantation, 76% showed further response after transplantation. In half of these responders, the disease state was changed into complete response (CR) after transplantation. 2-year overall survival was 52% and 2-year progressionfree survival was 36.8%. Median overall survival was 34 months (range 8–60), and median progression-free survival was 8 months (range 1–14). Median overall survival was 14 months (range 9–19) in the primary high-risk group (n=13), 7 months (range 4–10) in the resistance relapse group (n=5), and 6 months (range 0–14) in the primary refractory group (n=10). Overall survival in the sensitive relapse group (n=22) did not reach the median; the mean overall survival in this group was 33 months. The disease status before transplantation was the only significant prognostic factor in determining overall survival (p=0.032) and progression-free survival (p=0.001).
Conclusion
HDC and autologous PBSCT appears to produce high response rate. Primary high-risk group and sensitive relapse group had good prognosis, while refractory and resistance relapse group had poor prognosis. And the pre-transplantation disease status was the only significant prognostic factor in multivariate analysis.
INTRODUCTION
The intermediate-grade and high-grade non-Hodgkin’s lymphomas (NHLs) are highly chemosensitive malignancies, and even in patients with advanced disease, the complete response rate after chemotherapy is between 50 and 70%. Nevertheless, the cure rate is only in the order of 35% with the first-line chemotherapy regimen, CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), and patients who recur or do not respond require salvage therapy1, 2). Although a variety of salvage chemotherapy protocols have been developed, reported long-term survival rates range between 10–25%3, 9). Therefore, high-dose chemotherapy (HDC) and autologous peripheral stem cell transplantation (PBSCT) has been attempted in these groups of patients even after obtaining response to salvage treatment and in patients at a high risk of recurrence even in remission stage10). Long-term survival has been reported to be between 33–80% in sensitive relapse patients and 10–20% in the resistant relapse group11, 12). We investigated the initial stage, international prognostic index (IPI), response rate to HDC and autologous PBSCT, response duration, survival duration, toxicities, and prognostic factors related to the treatment.
MATERIALS AND METHODS
1. Patient selection
Between 1997 and 2002, 50 patients with non-Hodgkin’s lymphoma received HDC and autologous PBSCT. These patients were proven histologically to have non-Hodgkin’s lymphoma, and had relapsed or primary refractory disease responding to salvage therapy, or primary high-risk disease defined by clinical characteristics or histologic subtype. They were under 65 years of age with performance status from 0 to 2 by ECOG (Eastern Cooperative Oncology Group) criteria, and their liver, kidney, and heart functions (bilirubin: within 2.5 times normal level: creatinine, within 2.5 times normal level; and no history of heart failure or myocardial infarction in the past 3 months) were within normal limits. Patients with central nervous system involvement were excluded.
2. Peripheral stem cell collection
Peripheral blood stem cells (PBSC) were mobilized by using either salvage chemotherapy plus G-CSF or G-CSF only. Salvage chemotherapy regimens included IVAM and DHAP. In the salvage therapy plus G-CSF group, patients received 5 μg/kg/day of G-CSF (filgrastim) from the fifth day after chemotherapy until WBC count > 3,000/μL or CD34 cells were greater than 0.3%.
In the G-CSF only group, G-CSF treatment (10 μg/kg/day) was provided at a steady state for 6 consecutive days, and collection was performed between day 5–7. The goal was to collect ≥6×106 mononuclear cells/kg, or≥2×106 CD34+ cells/kg. Stem cells were kept frozen at −196°C until transplantation.
3. Treatment plan
Three different conditioning regimens were used. BEAM included; BCNU 300 mg/m2 iv, day −7; etoposide 200 mg/m2 iv, day −6–day 3; cytarabine 200 mg/m2 iv, day −6–day 3; and melphalan 140 mg/m2 iv, day −2. CMT included cyclophosphamide 50 mg/kg iv, day −7–day 6;, thiotepa 250 mg/m2 iv, day −5–day −4; and melphalan 50 mg/m2 iv, day −3–day −2. The high-dose chemotherapy regimen based on fludarabine which 9 patients used included fludarabine 50 mg/m2 iv for 2 days, and total body irradiation (1,000 cGy).
4. Response and toxicity evaluation
Treatment responses were divided into 4 stages according to the WHO guideline13). After autologous PBSCT, engraftment was confirmed by an increase of WBC to more than 1,000/L and an increase of platelet level to more than 50,000/μL. Toxicity was evaluated according to the standard World Health Organization (WHO) criteria14).
Pre-BMT status was defined as the following. Primary high-risk group included patients with an IPI score of more than 3 and histologic subtypes proven to be poor prognosis. Sensitive relapse group included relapsed patients who showed more than a partial response (PR) to salvage chemotherapy. Resistance relapse group included relapsed patients who did not respond to salvage chemotherapy. Primary refractory group included patients who did not respond to primary chemotherapy, but showed more than PR to salvage chemotherapy.
5. Statistical analysis
Progression-free survival was defined as the period from the time of stem cell infusion to either the time of disease progression or the time of final follow-up. Overall survival (OS) was defined as the period from the time of stem cells infusion to either the time of death or the time of final follow-up. Overall survival and time to progression were calculated using the Kaplan-Meier method. Univariate and multivariate analysis were done using SPSS’s log rank test and Cox regression on disease stage, lactic dehydrogenase (LDH) level, international prognostic index (IPI), bone marrow involvement, B symptoms, number of chemotherapy sessions done before conditioning regimens, and response to salvage therapy.
RESULTS
1. Patients’ characteristics
From July 1997 to January 2002, 50 patients were enrolled. There were 34 men and 16 women, with median age of 41 years (range: 14–61 years). Of the pathologic diagnosis, diffuse large-cell type was present in 23 patients, peripheral T-cell type in 5, anaplastic T-cell in 1, diffuse small cleaved-cell type in 2, NK/T-cell in 5, diffuse mixed small-& large-cell in 3, lymphoblastic type in 5, and mantle-cell type in 6 (Table 1). Nineteen patients were in CR, 26 were patients in PR, and 5 patients were in a refractory state before transplantation. Patient characteristics before transplantation are described in table 2.
2. Outcome and Response rate
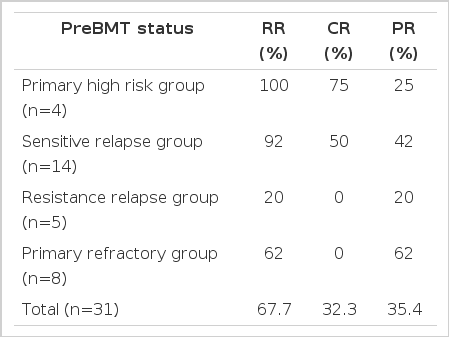
The median follow-up period in patients who underwent HDC and autologous PBSCT was 40.5 months (range: 9–61 months). The response rate after HDC and autologous PBSCT was 67.7% (32.3% complete response, 35.4% partial response) in patients are described in table 3 (n=31) who had evaluable lesions. The subgroup response rates in evaluable patients are described in table 3.
The 2-year overall survival rate was 52%, and the 2-year progression-free survival rate was 34%. The median overall survival was 34 months (range: 8–60 months), and the median progression-free survival was 8 months (range: 1–14 months, Figure 1).
In the primary high-risk group (n=13), the median overall survival was 14 months (range: 9–19 months), and the median progression-free survival was 16 months. In the sensitive relapse group (n=22), the overall survival did not reach the median survival, but the average was 33 months with the median progression-free survival of 14 months (range: 0–33months). In the primary refractory group (n=10), the median overall survival was 6 months (range: 0–14 months), and the median progression-free survival was 4 months (range: 0–9months). In the resistant relapse group (n=5), the median overall survival was 7 months (range: 4–10 months), and the median progression-free survival was 1 month. In the forty-five patients who responded to salvage therapy, the median overall survival was 34 months and the progression-free survival was 18 months (Figure 2).
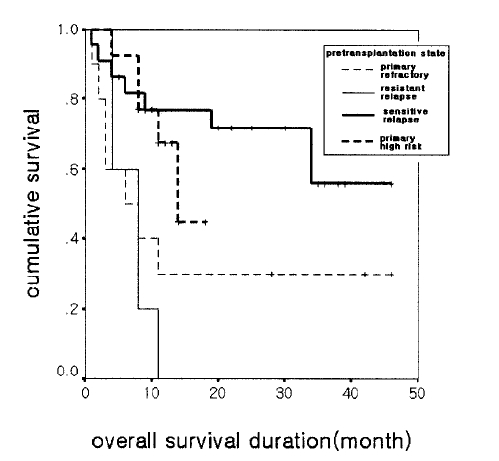
Overall survival according to the status of the disease at transplant: sensitive relapse, primary high-risk, primary refractory, and resistant relapse groups
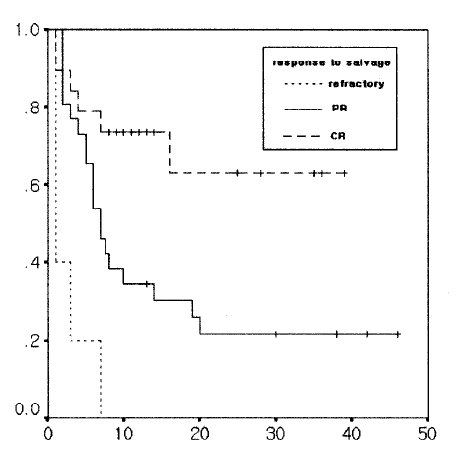
In patients who showed a complete response to salvage therapy, the overall survival and progression-free survival did not reach the median. In those who showed partial response to salvage therapy, the median overall survival was 14 months (range: 0–31 months), and the median progression-free survival was 7 months (range: 5–9 months), showing statistically significant difference in survival according to the log rank test (the overall survival rate: p =.006, progression-free survival rate: p=0001), (Figure 3).
3. Prognostic factors
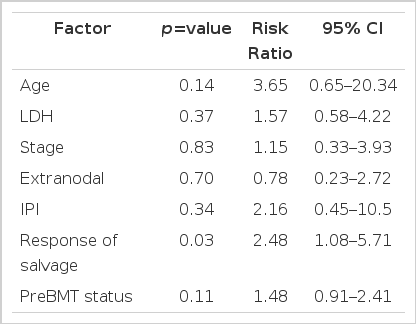
In multivariate analysis, response to salvage therapy was the only statistically significant factor influencing the overall survival rate (p=.032) and progressionfree survival (p=.001), (Table 4).
4. Engraftment and supportive care
After HDC, the median number of mononuclear cells infused was 10.2×108/kg (range: 2.0–595.0×108/kg), and the median number of CD34+ cell infused was 8.8×106/kg (range: 0.8–550×106/kg). To increase stem cell proliferation, 5 μg/kg of G-CSF was administered daily starting 72 h after peripheral stem cell administration. The median time to WBC count > 1000/μL was 10 days (range: 7–21 days), and a platelet count of > 50,000/μL was achieved at a median time of 20 days after transplantation (range: 12–172 days). No failure to engraft occurred and the patient who was reinfused with 0.8×106/kg CD34+ cell recovered the neutrophil count on day 15 after transplantation.
The median number of packed red cell transfusions was 3 times (range 1–7 times), the median number of platelet transfusions was 7 times (range 4–15 times), and the median number of G-CSF injections was 9 times (range 9–12 times). The median period of hospitalization was 36 days (range 21–76 days).
5. Toxicity
Treatment-related mortality occurred in three patients (6%) due to infection at D9, D19, and D66. Their most common non-hematologic toxicity was mucositis observed in 42% of the patients, but no side effect higher than grade 3 was observed. Nephrotoxicity was present in 1 patient (2%). Infection was seen in 10 patients, herpes zoster infection in 2, anal infection in 2, pneumonia in 2, external otitis in 1, and sepsis in 3 (Table 5).
DISCUSSION
Intermediate-grade and high-grade malignant lymphoma patients with primary high-risk and sensitive relapse disease indicated HDC and autologous PBSCT. Philip et al.15) reported the statistically significant difference in the 5-year survival rate between the patients who received conventional chemotherapy (32%) and those who underwent autologous BMT (53%) among intermediate-grade and high-grade malignant lymphoma patients with sensitive relapse disease. In the primary high-risk group, the 5-year disease-free survival rate was found to be 21% with conventional treatment. Haioun et al.16) proved that autologous bone marrow transplantation resulted in better survival than the conventional treatment in this group of patients. Based on these results, HDC using autologous PBSCT is believed to be more effective than conventional treatment in lymphoma patients with sensitive relapse and primary high-risk disease17, 18). As the conditioning regimen, we used BEAM, CMT and fludarabine-based regimens. Outcomes of autologous PBSCT using BEAM combined chemotherapy have already been reported in several studies. Caballero et al.19) reported an overall 3-year survival of 75% and 3-year disease-free survival of 65%. Mills et al.20) reported a 5-year survival of 41% and progression-free survival of 35%. From our center, Park et al.21) previously reported the 2-year survival of 41.2% and 2-year progression-free survival of 35.5%. The results reported by Mills et al. and Park et al. showed lower survival rates than those reported by Caballero et al. because patients with resistant relapse were included. CMT including cyclophosphamide, melphalan, and thiotepa was the conditioning regimen, which had been previously used at our center in autologous PBSCT for acute lymphocytic leukemia. In addition, Przepiorka et al.22) have already reported relatively fair results (52% CR and 26% PR) for HDC autologous PBSCT by using high doses of cyclophosphamide and thiotepa as the conditioning regimen in malignant lymphoma. In the present study, there was no difference in median survival (both 34 months) between the patients who used BEAM and those who used CMT chemotherapy, even though the BEAM group included 5 patients with resistant relapse. Since several studies have reported better results for the conditioning regimen that includes total body irradiation (TBI) than those without TBI, the use of TBI as conditioning regimen is expected to be gradually increased in the future23, 24).
According to the studies by Mills et al.20) and Park et al.21), better prognoseis are expected in the patients with sensitive relapse than resistant relapse and the best results are expected in patients with CR before transplantation. We were able to reconfirm the result of the previous reports in this study. Furthermore, multivariate analysis on various factors affecting the prognosis revealed that pre-transplantation disease status was the most important prognostic factor.
However, in sensitive relapse and primary refractory malignant lymphoma, the long-term survival is not more than 50%, even with HDC and autologous PBSCT. In order to overcome this problem, various methods including tandem high-dose chemotherapy, non-myeloablative allogeneic stem cell transplantation and immune therapy are being attempted. By using tandem high-dose chemotherapy, Fitoussi et al.25) reported the complete response rate of 58% in 24 patients with high-risk malignant non-Hodgkin’s lymphoma and Hodgkin’s lymphoma. However, further studies are needed because of deaths due to the high rate of toxicity and recurrence. Van Besien et al.26) examined allogeneic marrow transplantation in 64 patients with poor prognostic malignant lymphoma and reported that it was effective only in low-grade malignant lymphoma while showing a limited effect in intermediate-and high-grade malignant lymphomas. Moreover, Nagler et al.27) reported the 3-year survival rate and 3-year progression-free survival rate of 40% with allogeneic marrow transplantation using fludarabine chemotherapy in 23 patients with resistant malignant lymphoma. However, deaths due to graft-vs-host disease (GVHD) and infection posed a problem. Furthermore, immune therapy using Rituximab after HDC and autologous PBSCT has also been reported to show relatively encouraging outcomes in B-cell lymphoma including mantle cell lymphoma28).
In the present study, we reconfirmed that HDC and autologous PBSCT are effective for the treatment of recurrent and high-risk malignant lymphoma and the most important factor affecting the prognosis was pre-transplantation disease status.
Notes
This study was supported by a grant from the Catholic Cancer Center