Effects of Gender and Menstrual Cycle on Colonic Transit Time in Healthy Subjects
Article information
Abstract
Background :
Measuring colonic transit time (CTT) by the radio-opaque marker method is simple, widely available and important for the diagnosis of slow transit constipation. Moreover, the effects of gender and menstrual cycle on CTT remain controversial. Thus, in this study, we examined the effects of gender and menstrual cycle on CTT in healthy subjects.
Methods :
We measured CTT in 42 healthy subjects (21M, 21F) by using a radio-opaque marker, Kolomark™. Two simple abdominal radiographs were taken on the 4th and 7th days. Average daily intake of dietary fiber and menstrual history were surveyed.
Results :
The mean CTT of the 42 healthy subjects was 26.5±19.4 hours. The mean CTT was not significantly different between the male and female subjects (22.3±16.1 h vs. 30.1±21.4 h, p>0.05). However, the mean CTT of 11 female subjects in the luteal phase was significantly longer than that of 10 female subjects in the follicular phase (40.9±19.0 h vs. 20.6±19.2 h, p<0.05). Serum progesterone level, age, BMI, and the average daily intake of dietary fiber did not correlate with CTT.
Conclusion :
The effects of the menstrual cycle should be considered in interpreting CTT in young women.
INTRODUCTION
Idiopathic constipation is one of the most common functional digestive complaints. It can be divided into slow transit constipation and pelvic outlet obstruction, and colonic transit studies are necessary for their differential diagnosis.
Techniques of measuring colonic transit include the use of 1) non-absorbable markers, 2) colonic scintigraphy, and 3) radio-opaque markers. Techniques that involve non-absorbable markers, colored powders or chemically detectable markers, such as chromium oxide, are now obsolete because they are inconvenient, necessitating the- collection and examination of stools for several days. Colonic scintigraphy has become the method of choice for assessing colonic transit. However, this method requires skilled personnel and expensive facilities. Radiological techniques involving ingestion of radio-opaque markers of variable shapes or sizes have been devised for measuring colonic transit. These tests may require several abdominal radiographs taken for up to 7 days following marker ingestion. This process was simplified by taking one or two radiographs at the 4th and/or 7th day from marker ingestion1, 2). In Korea, the commonly used marker, Sitzmark™ (Konsyl Pharmaceuticals Inc., Texas, USA), has had limitations in its use due to high cost and difficulty in importation. However, recently, a domestically manufactured radio-opaque marker, Kolomark™, was introduced. Kolomark™ has been reported to be more radio-opaque and cheaper3). Until now, even though there were several reports of colonic transit studies using Kolomark™, it was thought that normative data on healthy Koreans were still lacking.
Despite longstanding disputes, it is not certain whether there is any difference in colonic transit time (CTT) between men and women, and whether CTT varies according to the menstrual cycle in women. Several studies have shown that CTT in women was longer than that in men4–9), while others10–13) did not find gender differences in CTT. Two previous studies8, 14) reported that the gastrointestinal (GI) transit time was more prolonged in the luteal phase than in the follicular phase; however, other studies7, 10, 15) did not show any statistical significance between the two phases.
Thus, we measured CTT in healthy subjects by using a radio-opaque marker, Kolomark™, and examined the effects of gender and menstrual cycle on CTT. Additionally, we investigated whether other factors including age, body mass index (BMI), and dietary fiber intake also affect CTT.
MATERIALS AND METHODS
1 Subjects
Forty two healthy subjects (21M, 21F) were recruited by intra-hospital advertisements. The ethical committee and institutional review board of our institution approved our study protocol. We obtained written consent to participate in the study from all the healthy subjects. Subjects were chosen from patients above 18 and below 60 years of age. None complained of GI symptoms including constipation and diarrhea, nor did any have a history of GI disease. GI symptoms were based on the Rome II criteria16). The other exclusion criteria were as follows: 1) pregnancy, 2) post-menopause, 3) previous abdomen operation except appendectomy or cesarean section, 4) having taken medicine known to affect GI motility during the previous one week, 5) diabetes mellitus, 6) other severe medical illnesses, 7) obesity with a BMI>30 kg/m2, and 8) alcoholism. Obese subjects were excluded because of a previous report17) that the colonic transit can be altered in this condition.
2 Methods
We had the subjects fill up a questionnaire about their bowel habits, stool frequency, GI symptoms, such as constipation, diarrhea, smoking, consumption of alcohol or coffee, menstrual cycle and last menstruatial period. We measured the subjects’ body weight and height with a Body Composition Analyzer (InBody 3.0, Biospace, Seoul, Korea). All the female subjects were confirmed to have a negative serum pregnancy test (Gravindex; Testpack™, Abbott, IL, USA) before the study.
The CTT was measured by using radio-opaque markers. The subjects each ingested one capsule that contained 20 radio-opaque markers (Kolomark™, M.I.Tech., Pyongtaik, Korea) in the morning at 24-hour intervals for three consecutive days, and two simple abdominal radiographs were taken at the supine position on the 4th and 7th days. Localization of markers on abdominal films relied on identifying bony structures as suggested by Arhan et al.18) The markers located to the right of the vertebral spinous processes above a line from the fifth vertebra to the right pelvic outlet were assigned to the right colon, markers to the left of the vertebral spinous processes and above an imaginary line from the fifth lumbar vertebra to the anterior superior iliac crest were assigned to the left colon, and markers inferior to a line from the pelvic brim on the right and the superior iliac crest on the left were judged to be in the recto-sigmoid colon and rectum. The total and segmental CTT’s were calculated as 1.2 times the sum of markers in the entire or segmental colon2, 5).
Subjects were asked to avoid unusually intensive physical activity and alcohol consumption, and to follow their usual daily lifestyles during the study. After having a dietitian give directions to the subjects, we had all the subjects keep their food diaries for three days and later analyzed them by using a food composition database and a software called as ‘Nutrition Anywhere ‘98’19), which had previously been recommended by the Korean Dietetic Association, and estimated the average daily amount of dietary intake of nutrients and fiber. Based on the information acquired from the questionnaires by the female subjects, we estimated whether they were in the luteal or follicular phase during the colonic transit study. To measure serum sex hormone levels in the female subjects, 10 mL of blood sampling was performed on the 1st day of the colon transit study and their sera were stored at −70°C until the measurement. Serum estradiol (Coat-a-count Estradiol DPC, Abbott, IL, USA) and progesterone (Coat-a-count Progesterone DPC, Abbott, IL, USA) levels were measured by radioimmunnoassay.
3 Statistical analysis
All the data were expressed as ‘mean±standard deviation (SD)’. Student’s t-test, Mann-Whitney U test and Pearson’s correlation coefficient were used in the analysis as appropriate. The analysis of the data was performed with a statistic package SPSS/PC window 11.0 program (Statistical Package for the Social Science, SPSS Inc., Chicago, IL, USA). Values of p less than 0.05 were regarded as ‘significant’.
RESULTS
1 Comparison of baseline characteristics and dietary intake according to gender
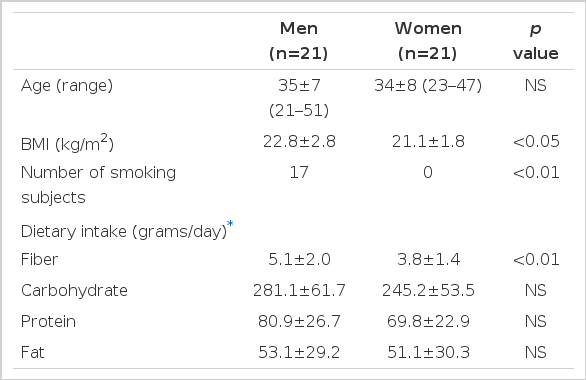
The mean age of the 42 healthy subjects was 34±7 (range: 21–51). BMI was higher in the male subjects than in the female subjects (p<0.05). The smoking frequency was higher in the male subjects than in the female subjects (17/21 vs. 0/21, p<0.01). The average dietary fiber intake was significantly less in the female subjects than in the male subjects (3.8 vs. 5.1 grams/day, p<0.01). However, the average dietary intake of other nutrients including carbohydrate, protein and fat were not significantly different between the male subjects and the female subjects (Table 1).
2 Comparison of CTT according to gender
The mean total CTT of the 42 healthy subjects was 26.5±19.4 hours (h). The upper limit of total CTT, described as ‘mean+2SD’, was 65.3 h: 54.5 h in the male subjects and 72.9 h in the female subjects. The mean total CTT of the male subjects (22.3±16.1 h) was shorter than that of the female subjects (30.1±21.4 h), but without statistical significance (Figure 1). Comparing segmental CTT’s between the male and female subjects, the left CTT was significantly longer in the female subjects (12.5±10.7 h) than in the male subjects (6.0±6.1 h, p<0.05). However, the right CTT and recto-sigmoid CTT were not different between the male and female subjects (Table 2).
3 Comparison of CTT between luteal phase and follicular phase of the menstrual cycle
10 female subjects were in the luteal phase and 11 were in the follicular phase during the colonic transit study. The mean total CTT of the female subjects in the luteal phase (40.9±19.0 h) was significantly longer than that of those in the follicular phase (20.6±19.2 h, p<0.05). Among the segmental CTT’s, the recto-sigmoid CTT was significantly longer in the female subjects in the luteal phase (18.7±11.0 h) than in those in the follicular phase (6.1±7.9 h, p<0.05) (Table 2).
Comparing baseline characteristics between the female subjects in the luteal phase and those in the follicular phase, there were no significant differences in mean age, BMI, and daily dietary intake of fiber. The mean serum progesterone level of the female subjects in the luteal phase (4.9±5.0 ng/mL) was significantly higher than that of those in the follicular phase (2.2±4.0 ng/mL, p<0.05), while there was no significant difference of the mean serum estradiole level between the two groups (Table 3).
4 Correlations between clinical variables and CTT
Age, BMI, daily dietary intake of fiber, serum progesterone level, and serum estradiole level did not show significant correlation with the total and segmental CTT’s except that the amount of dietary fiber intake had a positive correlation with right CTT (r=0.393, p=0.01), and the serum estradiole level had a positive correlation with recto-sigmoid CTT (r=0.460, p=0.04) (Table 4).
DISCUSSION
When we measured the CTT of the 42 healthy subjects using Kolomark™, a domestically manufactured radio-opaque marker, the average time was 26.5 h, which was similar to previous studies that had used Sitzmark™4, 11). The reported CTT’s of healthy Koreans seem to be shorter than those of Westerners: two Western reports showed 35.0 h2) and 33.4 h20). This difference of CTT might be caused by different dietary habits, lifestyles or genetic backgrounds, but requires further study.
In our study, the upper normal limit of CTT, defined as ‘mean+2 SD’, was 65.3 h: 54.5 h in the male subjects and 72.9 h in the female subjects. Further validation studies are needed whether those values can be used as useful reference values for the diagnosis of slow transit constipation.
It is widely known that the prevalence of constipation is higher in women than in men21), and in particular, it becomes more prominent in women of childbearing age, suggesting a role of the female sex hormone22). However, the gender difference of CTT is still controversial: some have suggested longer mean CTT in women4–9), while others have indicated no significant difference between men and women10–13). In our study, the mean total CTT was 22.3±16.1 h in the male subjects and 30.1±21.4 h in the female subjects: as in two previous Korean studies10, 11), there was no significant difference in the total CTT between the male and female subjects. Our lack of statistical significance might be attributable to the small sample size of our study over the large biological variation of CTT, or the different frequency of smoking subjects between the male subjects and the female subjects. In our study, the smoking frequency was significantly higher in the male subjects (17/21) than in the female subjects (0/21). Since CTT in non-smoking males was previously reported8) to be significantly shorter compared with smoking males, future studies are suggested after adjusting to smoking frequency.
The radio-opaque marker study can also provide us with information about the segmental CTT. Our study showed that the left CTT of the female subjects was significantly longer than that of the male subjects, which was similar to a previous report9). However, another report5) showed that the right CTT was significantly longer in the female subjects. A recent study23) that used ambulatory 24-h colonic manometry revealed that women showed less pressure activity than men, and this difference was particularly significant in the transverse/descending colon. Therefore, the gender difference of segmental colonic motility might contribute to the prevalence of constipation in women, but more studies on its mechanisms are required.
Female sex hormones can affect colonic motility: the CTT was markedly prolonged in the second or third trimester of pregnancy when serum progesterone levels are high24), and in a vitro study, progesterone had a dose-dependent, inhibitory effect on the contraction of intestinal smooth muscles. Progesterone was reported to modulate intracellular or transmembranous calcium transportation25, 26) or antagonize the effect of motilin, a gut stimulating hormone27). We can hypothesize that CTT will vary according to the menstrual cycle: the CTT gets longer in the luteal phase, when the serum progesterone level is high, and vice versa in the follicular phase. However, the hypothesis was not proved by previous studies5, 7). In our study, both the total and the recto-sigmoid CTT’s of the female subjects in the luteal phase were significantly longer than those of the female subjects in the follicular phase, which was similar to results obtained by Meier et al.8) and Davies et al28). When we compared the baseline characteristics between the female subjects in the luteal phase and those in the follicular phase, they were not different with each other except that the mean serum progesterone level was higher in those in the luteal phase than in those in the follicular phase. Therefore, it was thought that progesterone might have played a role in prolonging CTT in the female subjects in the luteal phase. However, we did not find any significant correlation between the serum progesterone level and CTT. Future studies, such as repeating CTT’s in the same subjects according to the menstrual cycle or defining the menstrual cycle more objectively by confirming ovulation by sonography, are needed.
Other factors that can influence CTT are age, BMI, and dietary fiber2, 4, 5, 8, 12). A previous study12) showed that the CTT of healthy older subjects (age 55–74 years) had a longer mean CTT than healthy young subjects (age 21–27 years). However, in our study, age did not correlate with CTT at the age range of 21 to 51 years. In addition, BMI did not correlate with CTT, as in previous studies4, 29). However, subjects with a BMI over 30 kg/m2 were excluded.
As the amount of dietary fiber increased, the mean CTT was shortened and the stool frequency increased28): the colonic transit of vegetarians was faster than that of omnivores, and dietary fiber was effective in reducing CTT28, 30). However, in our study, CTT did not correlate with the amount of dietary fiber intake, which was similar to another study4). This discrepancy could be due to the limitation of methods in measuring the amount of dietary fiber intake based on the subjects’ food diaries. Although the absolute amount of the dietary fiber intake of our study subjects was similar to a previous study31), by the Korean Ministry of Health and Welfare, the calculated method19) measured only the crude fiber content rather than total fiber. Crude fiber is a remnant after the total fiber is processed with acid or base, and measuring the total fiber is considered extremely difficult.
In conclusion, the mean total CTT of the 42 healthy subjects was 26.5 h. Although there was no difference in total CTT between the male and female subjects, the mean total CTT of the female subjects in the luteal phase was significantly longer than that of the female subjects in the follicular phase. Age, BMI, and the average daily intake of dietary fiber did not affect total CTT.
Acknowledgements
This work was supported by a grant from the Clinical Research Fund of Ewha Womans University Tongdaemun Hospital.