Long-Term Clinical Benefits of a Platelet Glycoprotein IIb/IIIa Receptor Blocker, Abciximab (ReoPro®), in High-Risk Diabetic Patients undergoing Percutaneous Coronary Intervention
Article information
Abstract
Background
High-risk percutaneous coronary interventions (PCIs) are associated with a high complication rate, a low procedural success rate and a high restenosis rate, especially in diabetics. We sought to determine whether abciximab (ReoPro®) therapy affects long-term clinical outcomes of Korean patients with diabetes undergoing high-risk PCI.
Methods:
One hundred and nineteen patients with 152 lesion sites were administered ReoPro® among 2,231 patients who underwent PCI at Chonnam National University Hospital from March 1999 to Feb 2001. These 119 patients were divided into two groups, 30 were allocated to a diabetic group (Group I, 57.7±8.2 years, 22 male), and 89 to a non-diabetic group (Group II, 59.6+10.8 years, 68 male). Early and long-term clinical outcomes after PCI were analyzed.
Results:
In terms of clinical diagnosis, the number of acute myocardial infarctions in Group I was 25 (83.3%) and 76 in Group II (85.4%). As for risk factors, target artery lesions, and ACC/AHA types, no differences were found between the two groups. The number of patients with total occlusion was 21 (55.3%) and 62 (53.9%), and the number with a thrombus-containing lesion was 28 (93.3%) and 88 (98.9%) in Groups I and II, respectively. The procedure was successful in 27 (90.0%) in Group I, and in 80 (89.9%) in Group II, and no differences were evident between the two groups in terms of bleeding complications. No major adverse cardiac events (MACE), including myocardial infarction, repeat revascularization or cardiac death, were observed in Group I, but 8 cases of MACE occurred in Group II during hospitalization. Clinical follow-up was performed in 116 patients (97.5%) over 18.5 ± 6.7 (5–28) months. The number of overall MACEs was 10 (3.3%) in Group I and 14 (15.7%) in Group II (p=0.038).
Conclusion:
ReoPro® used in high-risk PCI in diabetics was effective in terms of early clinical outcomes, but its long-term clinical benefits were not proven.
INTRODUCTION
Percutaneous coronary intervention (PCI) has greatly contributed to the symptomatic relief and to the improvement of quality of life of coronary artery disease patients, and it is now firmly established as a major treatment for ischemic heart disease. However, acute coronary occlusion occurs in 4 to 9% of all PCIs during or immediately after the procedure, and it is an important cause of post-PCI mortality and morbidity and revascularization by, for example, emergency coronary bypass surgery1, 2). Platelet-mediated thrombosis is known to play a critical role not only in the pathophysiology of acute coronary syndrome but also in the pathogenesis of acute coronary occlusion3, 4). Although anti-platelet agents such as aspirin reduce post-PCI acute coronary occlusion5, 6), high-risk PCI still harbors complication rates of up to 10 to 20%7).
A platelet glycoprotein IIb/IIIa receptor blocker, Abciximab (ReoPro®), is a potent anti-platelet agent that blocks the final common pathway of platelet aggregation. Moreover, it has proven to be effective in improving results of high-risk PCIs, and in reducing the incidence of major adverse cardiac events (MACEs), including short- and long-term mortalities. Such findings promote its routine use in high-risk PCI8–11). Park et al12) in 1998, and Kim et al13) in 2001, investigated short- and long-term clinical effects of ReoPro® in a Korean population, and reported that it could be used relatively safely and effectively in patients with acute myocardial infarction (AMI) undergoing thrombus-containing high-risk PCI without major hemorrhagic complications.
Diabetic patients have a higher mortality rate after both percutaneous and surgical coronary revascularization than non-diabetic patients14, 15). Even in this era of stenting, diabetics have worse outcomes than non-diabetics16).
In this study, we sought to evaluate long-term clinical benefits and safety of abciximab (ReoPro®), a platelet glycoprotein IIb/IIIa receptor blocker, in diabetics undergoing high-risk PCI.
MATERIALS AND METHODS
1. Patients
Of 2,231 patients who underwent PCI at Chonnam National University Hospital between March 1999 and February 2001, one hundred and nineteen patients (90 males, 29 females, 59.1±10.8 years of age) with 152 lesions, regarded as high risk for the presence of intracoronary thrombus and administered ReoPro® at the time of PCI, were assigned to the diabetic or the non-diabetic group. Group I consisted of 30 diabetics with 38 lesions (21 males, 57.7±8.2 years of age) and group II of 89 non-diabetics with 114 lesions (69 males, 59.6±10.8 years of age).
2. Definition of Diabetes
Patients were classified as diabetic if they were receiving oral hypoglycemic or insulin therapy at the time of hospital admission, or if they had been previously found to have a fasting blood glucose concentration at or above 140 mg/dL.
3. Definition of High-risk group
Patients at high risk for abrupt vessel closure were defined as those with an intracoronary thrombus or a total thrombotic occlusion by coronary angiography, which necessitated direct or rescue percutaneous intervention, according to the criteria of the American College of Cardiology/American Heart Association (ACC/AHA)18) and that of the EPIC investigators8). High risk patients who underwent PCI with rescue use of ReoPro® were eligible for enrollment in the study. Patients were excluded if they were known to have a bleeding diathesis, had undergone major surgery within the preceding six weeks, or had experienced a stroke during the preceding two years.
4. Methods
All patients were treated with an oral dose of aspirin (100–200 mg) and ticlopidine (500 mg) before angioplasty; aspirin was administered continuously and ticlopidine was prescribed for one month after the procedure. In the case of patients with acute myocardial infarction (AMI) aspirin was given at an initial oral dose of 300 mg at the time of arrival at the emergency room, and this was followed by a dose of 200 mg per day thereafter.
Heparin was given intravenously as an initial bolus dose of 5,000 units followed by a continuous infusion of 1,000 units to maintain the activated partial thromboplastin time (aPTT) at 2.0 to 2.5 times the control value. A bolus dose of 5,000 units was also administered just before the PCI to maintain the activated clotting time of between 300 and 350 seconds over the duration of the procedure. When dalteparin, a low-molecular-weight heparin was used, an initial bolus of 2,500 units was given as vascular sheaths were advanced and an additional dose of 5,000 to 7,000 units during angioplasty.
Abciximab (ReoPro®), the chimeric 7E3 Fab (Centocor BV, Leiden, Netherlands), used in the present study, is a Fab fragment of a human-mouse genetic reconstruction of a murine monoclonal IgG molecule that binds selectively to the glycoprotein IIb/IIIa platelet receptor. When patients were considered to have a high risk of vessel closure they were administered ReoPro® as a bolus of 0.25 mg/kg at the time of PCI, and this was followed by 10 μg per minute infusion for 12 hours after PCI.
Vascular access sheaths were maintained for at least six hours after infusion of the study drug, and were left in place for at least five hours after the heparin infusion, until an acceptable activated partial-thromboplastin time (<50 seconds) was achieved to maintain hemostasis. After sheath removal, manual compression was applied for hemostasis for at least thirty minutes by an experienced technician and an absolute bed rest was advised for a minimum of 6 hours after the procedure.
Blood samples were obtained before and 1 and 24 hours after the study drug administration and then daily until hospital discharge. The samples were carefully examined for evidence of changes in blood cell counts and other laboratory abnormalities.
Follow-up coronary arteriography was performed six-months after PCI. Significant stenosis was defined as a narrowing of more than 50 percent in the main coronary artery or its major bifurcations. Angiographic analyses were performed on end-diastolic frames of angiograms obtained before and after PCI and measurements were made in the projection showing the most severe stenosis, using an electronic caliper or Phillips H5000 DCI program. Successful PCI was defined as a residual diameter narrowing of <30% with TIMI (Thrombolysis in Myocardial Infarction) flow >II and no complications, such as acute myocardial infarction, emergency percutaneous revascularization, emergency surgical bypass, or death.
Bleeding events were classified as major, minor, or insignificant, according to the criteria of the Thrombolysis in Myocardial Infarction Study Group14). Major bleeding events were defined as intracranial bleeding or a bleeding event that caused a decrease in hemoglobin of >4 g/dL or required transfusion of >3 units of blood. Minor bleeding was defined as bleeding resulting in a hemoglobin decrease of <4 g/dL or that which required transfusion of <3 units of blood. Thrombocytopenia was defined as a platelet count of <100×109/L.
Clinical and coronary angiographic data were obtained and analyzed retrospectively by reviewing medical records and databases. Clinical follow-up was made by telephone interview with the patients and by review of the medical records.
Patients were screened for the major risk factors for coronary artery disease, namely hypertension (>140/90 mmHg), hypercholesterolemia (serum total cholesterol level >240 mg/dL), smoking, and diabetes by medical history taking, physical examination, and laboratory findings. The primary end-point was a composite of death, myocardial infarction, or severe myocardial ischemia requiring urgent surgical or repeated percutaneous coronary revascularization. The incidences of major adverse cardiac events (MACEs) such as death, acute myocardial infarction, and revascularization were assessed during the clinical follow-up.
5. Statistical analysis
Statistical analyses were performed using SPSS for Windows, Release 10.0. Group data are expressed as mean values ± standard deviation for continuous variables or as percent frequencies for categorical variables. Group differences were assessed by using Chi-square analysis for discrete variables and the unpaired sample t-test for continuous variables. A p value of less than 0.05 was considered statistically significant.
RESULTS
1. Baseline Clinical Characteristics
In terms of sex and age, group I consisted of 21 males (70.0%) of mean age 57.7±8.2 years, and group II consisted of 69 males (77.5%) of mean age 59.6±10.8 years, which were not significantly different. Clinical diagnoses did not differ in the two groups, acute myocardial infarction was the most common: 25 patients (83.3%) and 76 patients (85.4%) in group I and II, respectively. In terms of the risk factors of coronary artery disease, hypertension accounted for 15 patients (50.0%) in group I and 31 patients (34.8%) in group II, hyperlipidemia for 8 patients (26.7%) in group I and 18 patients (20.2%) in group II, and smoking in 14 patients (46.7%) in group I and 55 patients (61.8%) in group II. No significant differences were found between the groups in terms of these risk factors. Three patients (10.0%) in group I and 4 patients (4.5%) in group II experienced non-hemorrhagic cerebrovascular accidents, 3 patients (10.0%) in group I and 4 patients (4.5%) in group II experienced a transient ischemic attack, and 1 patient (3.3%) in group I and 3 patients (3.4%) in group II suffered peripheral vascular disease. These results were not significantly different in the two groups. The number of patients that had previously undergone PCI was 2 (6.7%) in group I and 5 (5.6%) in group II, and the number of patients who had undergone coronary artery bypass surgery was 1 in each group; again these results were not significantly different. By echocardiographic analysis left ventricular ejection fraction by Simpson’s formula was 44.1 ±12.6% in group I and 46.3±10.7% in group II, and the number of patients with an ejection fraction of less than 40% was 8 (26.6%) in group I and 26 (29.2%) in group II. The number of patients with acute myocardial infarction who had undergone thrombolysis prior to PCI was 6 (20.0%) in group I and 19 (21.3%) in group II (Table 1).
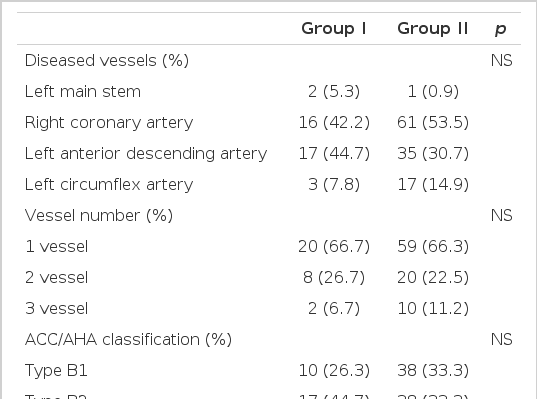
2. Angiographic Findings
With regard to the number of vessels with lesions, single-vessel disease accounted for 20 patients (66.7%) in group I and 59 patients (66.3%) in group II, two-vessel disease for 8 (26.7%) in group I and 20 (22.5%) in group II, which were not significantly different. In terms of the diseased vessel, the culprit lesion was in the left main stem in 2 (5.3%) patients in group I and 1 (0.9%) in group II, in the right coronary artery in 16 (42.4%) in group I and 61 (53.5%) in group II, in the left anterior descending artery in 17 (44%) in group I and 35 (30.7%) in group II, and in the left circumflex artery in 3 (7.8%) in group I and 17 (14.9%) in group II. According to the criteria of ACC/AHA, type B1 lesions were 10 (26.3%) in group I and 38 (33.3%) in group II, type B2 lesions 17 (44.7%) in group I and 38 (33.3%) in group II, and type C lesions 11 (28.9%) in group I and 37 (32.5%) in group II.
The reference diameters were 3.08±0.21 mm in group I and 3.05 ±0.28 mm in group II, which were not significant. As to TIMI flow and stenosis prior to PCI, lesions with TIMI flow 0 were the most common, being 21 (55.3%) in group I and 62 (54.4%) in group II; and TIMI flow 2 lesions were 8 (21.1%) in group I and 25 (21.9%) in group II.
Balloon angioplasty was performed in 16 lesions (42.2%) in group I and in 47 lesions (41.2%) in group II, and stenting was performed in 22 lesions (57.9%) in group I and 67 lesions (58.8%) in group II. Suboptimal lesions were the most common indication for stenting, and there were 14 (60.9%) in group I and 35 (53.0%) in group II. Abrupt occlusion occurred in 9 lesions (39.1%) in group I and in 32 lesions (45.4%) in group II. Stent length was 17.1±4.5 mm in group I and 17.4±4.6 mm in group II, which was not significantly different. In terms of the types of stents used in PCI, MAC stents were the most commonly used stents, being used in 10 cases (43.5%) in group I and in 31 cases (47.0%) in group II, NIR stents were used in 7 cases (30.4%) in group I and in 13 cases (19,7%) in group II, and CrossFlex stents in 3 cases (13.0%) in group I and 9 cases (13.6%) in group II. These differences were not significant (Table 2).
3. Results of PCI and in-hospital Major Adverse Cardiac Events (MACE)
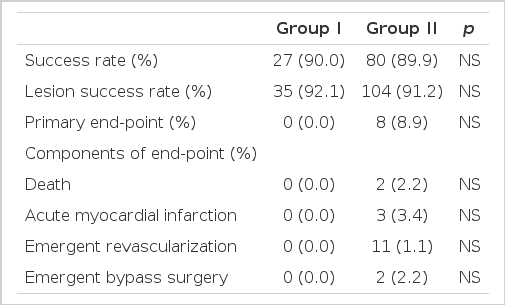
The rate of successful PCI was 90.0% (27 lesions) in group I and 89.9% (80 lesions) in group II, and the rate of lesion success was 92.1% (35 lesions) in group I and 91.2% (104 lesions) in group II. Complications related to PCI occurred in 4 patients (10.5%) in group I and in 10 patients (8.7%) in group II. Acute thrombotic occlusion occurred in 1 patient (2.6%) in group I and in 2 patients (1.8%) in group II, no-reflow phenomenon in 2 patients (5.2%) in group I and in 5 patients (4.4%) in group II, closure of bifurcation sites in 1 patient (2.6%) in group II, guidewire crossing failure in 1 patient (2.6%) in group I and 2 patients (1.8%) in group II.
The composite primary end point (in-hospital death, acute myocardial infarction, emergency percutaneous coronary angioplasty, or emergency coronary artery bypass surgery) occurred in 8 patients (9.0%) in group II, but there was no significant difference between the groups (p=0.089). MACE did not occur in group I. Among the total of 119 patients death occurred in 2 (2.2%), acute myocardial infarction in 3 (3.4%), emergency percutaneous coronary angioplasty in one (1.1%), and coronary artery bypass surgery in two (2.2%) (Table 3).
Both deaths occured in AMI patients with left ventricular ejection fraction of 35% or below one patient died of ventricular fibrillation which developed 12 hours after PCI and did not respond to any intensive treatment including DC counter-shocks, and the other died suddenly of ventricular arrhythmia during recovery.
4. Bleeding Complications
Major bleeding occurred in 2 patients (2.2%) in group II. In one patient, it was due to recurrence of a bladder cancer and required transfusion of 7 pints of blood. The other patient suffered gastric ulcer bleeding and recovered after receiving 7 pints of blood. Cerebral hemorrhage did not occur in either group. Minor bleeding occurred in 2 patients (2.2%) with peptic ulcer in group II in association with use of non-steroidal anti-inflammatory drugs, but improved after transfusion of 2 pints of blood. As to the site of bleeding complications a hematoma of less than 5 cm developed at the site of vascular sheath insertion in one patient (3.3%) in group I and in 6 patients (6.7%) in group II. Other complications that occurred in group II, included a hematoma greater than 5 cm at the vascular sheath insertion site in one patient (1.1%), gingival bleeding in 2 patients (2.2%), gastrointestinal bleeding in 3 patients (3.4%), and urinary tract bleeding (e.g., hematuria) in 3 patients (3.4%). The incidence of total hemorrhagic complications was 3.3% (n=1) in group I and 15.7% (n=14) in group II, which were not significantly different (p=0.07). All bleeding events improved with conservative treatment without any sequelae. Thrombocytopenia was associated with the use of ReoPro® in one patient (1.1%) in group II (Table 4).
5. Long-term Clinical Outcome
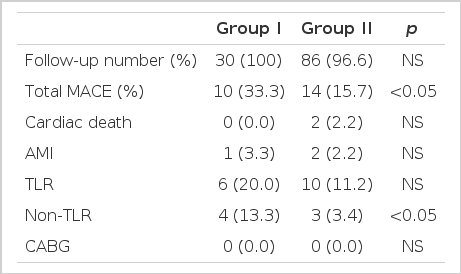
A total of 116 patients (97.5%) were followed up for a period of 18.5±6.7 (5–28) months. During this period death occurred in 2 patients (2.2%) in group II, acute myocardial infarction in one patient (3.3%) in group I and in 2 (2.2%) patients in group II, target vessel revascularization in 6 patients (20.0%) in group I and in 10 patients (11.2%) in group II, and non-target vessel revascularization in 4 patients (13.3%) in group I and in 3 patients (3.4%) in group II (p=0.045). The incidence of total major adverse cardiac events was 33.3% (n=10) in group I and 15.7% (n=14) in group II (p=0.038) (Table 5).
DISCUSSION
Platelet glycoprotein (GP) IIb/IIIa receptor inhibitors are the most potent anti-platelet agents, and block the final common pathway of platelet aggregation. In the formation of intracoronary thrombus the GP IIb/IIIa receptor is activated in platelets by collagen, thrombin and adenosine diphosphate. It then binds circulating fibrinogen and von Willebrand factor, which cross-link receptors on adjacent platelets, leading to platelet aggregation and thrombus formation18, 19). Since the GP IIb/IIIa receptor is the most important factor in the development of acute intracoronary thrombus, it seems that inhibition of fibrinogen binding at this receptor on the platelet surface could reduce the formation of platelet-rich thrombus by blocking platelet interactions19).
Representative GP IIb/IIIa receptor blockers currently in clinical use are abciximab (ReoPro®, c7E3 Fab), a chimeric human-murine monoclonal antibody fragment; eptifibatide (Integrilin®), a peptide; and Tirofiban (Agrastat®) and Lamifiban, which are peptidimimetics8–11). Among these, unlike the low molecular weight GP IIb/IIIa receptor blockers, ReoPro® also binds with equivalent affinity to the vitronectin receptor, present on smooth muscle cells, platelets, and vascular endothelial cells. Moreover, the blockade of the vitronectin receptor is believed to prevent restenosis after acute vessel injury by inhibiting migration and proliferation of smooth muscle cells18–20).
In four large-scale placebo-controlled trials the blockade of the platelet surface membrane glycoprotein IIb/IIIa complex by abciximab (ReoPro®), a human-murine chimeric antibody Fab fragment, has proven to markedly reduce the incidence of acute ischemic complications in the setting of percutaneous coronary revascularization8–10, 21). Particularly, in patients undergoing high-risk PCI, Abciximab (ReoPro®), has been shown to reduce the risk of early ischemic complications8) and the incidence of major adverse cardiac events during the 6 months after PCI22). The EPILOG trial revealed that use of ReoPro® combined with low-dose heparin is safe, and indicated that the effect of ReoPro® on risk reduction could allow its use to be extended to patients undergoing routine elective PCI9).
For patients with a complex coronary anatomy, stents and ReoPro® are believed to confer additive long-term benefits with respect to survival, MI, and target vessel revascularization (TVR). In addition, relative benefits of the adjunctive use of ReoPro® to reduce ischemic events appear to be more pronounced among patients with complex rather than simple lesions23).
Little is known about ReoPro®’s preventive effects upon restenosis after PCI. It was reported to be effective in diabetics in the EPISTENT trial21), while the ERASER study24) found no benefit in terms of reducing in-stent restenosis.
Diabetes is an important prognostic predictor in patients undergoing PCI, and a major risk factor of coronary artery disease. Diabetic patients have been reported to have an increased risk of procedure-related complications during hospitalization and higher rates of recurrence and decreased infarct-free survival after hospital discharge25). In terms of long-term follow-up, diabetic patients undergoing percutaneous rather than surgical revascularization showed nearly a threefold increase in the late mortality26).
To explain these findings a variety of reports have suggested that perturbations in the coagulation mechanism and of platelet activity occur in diabetics27–30). Abnormalities in the coagulation systems of diabetic patients that might affect platelet function include larger platelets27), the greater expression of P-selectin33), a higher platelet surface density of GP IIb/IIIa28, 29), altered thromboxane metabolism34), and higher levels of circulating fibrinogen29), vitronectin35, 36), and thrombin-antithrombin III complexes37), as well as more extensive endothelial dysfunction31, 32). These factors are known to indicate a poor clinical outcome.
Diabetic patients suffer higher mortality from acute coronary syndromes and in general are more at risk of extensive coronary artery disease than non-diabetics. It has also been reported that diabetic patients are more likely to have plaque ulceration and evidence of a red or fibrin-rich thrombus38) than non-diabetic patients.
An analysis of the characteristics and outcomes of diabetics enrolled in a large multi-center study (EPILOG) revealed that ReoPro® treatment led to a reduced composite of death and myocardial infarction, which was at least as great as that seen in non-diabetic patients. On the other hand, target vessel revascularization was reduced in non-diabetics but not in diabetics39). This study demonstrated that diabetics had a higher incidence of both target and non-target lesion revascularization, but that only non-target lesion revascularization was a statistically significant variable, which is contrary to the findings of the majority of other similar studies. This discrepancy could be explained in part by the fact that only patients with a high-risk of acute thrombotic occlusion were selected for the study. Although this study revealed higher incidence of target-lesion revascularization in the diabetic patients, it failed to prove significant difference between the groups, deserving further investigation into the efficacy of GP IIb/IIIa inhibitors against restenosis. Moreover, the rate of target vessel revascularization in diabetic patients was considerably lower than that seen previously in diabetics. This result may in part be explained by the fact that advances in the performance of PCI, probably because of better imaging, better dilation catheters, and provisional stent implantation, have reduced complications and recurrence rates after PCI, leading to the failure of ReoPro® to further reduce the rate of revascularization in diabetics. It is also possible that the enhanced early protection afforded by ReoPro® against the composite of death and myocardial infarction may have led to a paradoxical increase in revascularization procedures by providing a larger pool of survivors with viable myocardium. In addition, a higher density of GP IIb/IIIa receptors on platelet surfaces in diabetic patients might leave less ReoPro® available for binding to smooth muscle vitronectin receptors, and therefore, this would result in a relative increase in the rate of restenosis after PCI. The incidence of non-target vessel revascularization was, however, significantly higher in the diabetic group, suggesting that the use of ReoPro® could not provide a long-term benefit with regard to the progression of atherosclerosis or alterations in the platelet and endothelial functions of patients with diabetes.
The mechanism of ReoPro®’s benefit in the reduction of mortality in diabetics is unclear. It is generally accepted that in patients with diabetes the coagulation cascade is altered and that their response to arterial injury following treatment with a drug or procedure is perverted, which increases the propensity of adverse events. In addition, the microvasculature of diabetics is often diffusely diseased and may be poorly suited to handle the thromboembolic burden created by PCI, and perhaps the instrumentation of an artery and the microscopic nature of an injury contribute to new lesion development40). In this regards, although the present study has limitations in that it is neither large-scale nor prospective, it is noteworthy that no differences in the procedural or bleeding complications were observed between the diabetic and nondiabetic patients. This may be explained by the periprocedural ReoPro®’s attenuation of endothelial response to such injury through its binding to receptors other than the GP IIb/IIIa receptor41). ReoPro®’s possible benefit in reducing neointimal growth, as documented by angiography in stented patients, provides another mechanism for its effects42–46).
One of the main limitations of our study is that it is not a double-blind, controlled, prospective study and therefore can not be an absolute comparison on ReoPro®. Our findings warrant that a more comprehensive study be undertaken. Furthermore, only patients with an intracoronary thrombus who developed complete vessel closure immediately after PCI were considered eligible, according to the rules of medical insurance coverage in Korea. This differs from the criteria of high-risk features established and used by foreign countries, which include for example, myocardial infarction evolution within 12 hours of the onset of symptoms that necessitate direct or rescue PCI, early postinfarction angina, or unstable angina not responding to medical therapy. In addition, classification, duration, and the extent of control of diabetes were not considered.
This study shows that ReoPro®, a GP IIb/IIIa receptor blocker, used at the time of PCI, is safe and does not increase the risk of serious bleeding complications in diabetics with a high-risk profile. However, ReoPro® did not confer pronounced long-term benefits with respect to target vessel revascularization.