INTRODUCTION
Asthma is characterized by chronic inflammation of the airways, associated with inflammatory cells, including eosinophils, mast cells, basophils, macrophages, and T helper (TH) cells. These cells are involved in development of airway hyperresponsiveness (AHR), bronchoconstriction, mucus secretion, and remodeling by releasing inflammatory mediators, such as chemokines, growth factors, lipid mediators, and chemical mediators. Then, the complex pathogenesis of asthma is contributed to by various cellular responses, based on the dysregulated interaction between the innate and adaptive immune systems.
Recent evidence suggests that prostaglandin D2 (PGD2), a major prostanoid, may play an important role in orchestrating interactions between mast cells, TH2 cells, eosinophils, and dendritic cells.
TH2 immune response in asthma
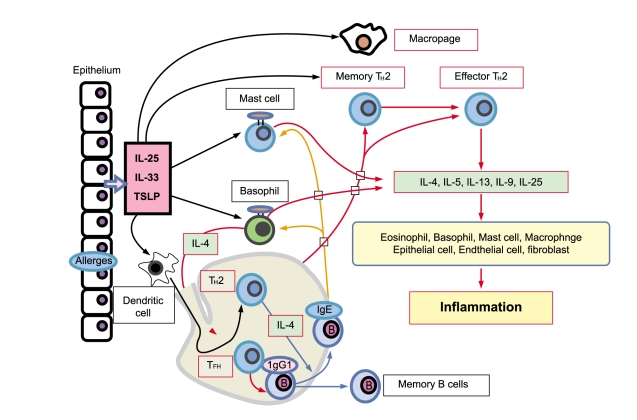
Allergic diseases are characterized by elevated serum immunoglobulin (Ig) E levels and hypersensitivity to normally innocuous antigens as allergens. A particular allergen first encounters antigen-processing cells (APC), such as dendritic cells or macrophages, directly. The allergen captured by the APC is processed and presented to CD4+ T cells. CD4+ T cells are polarized into distinct types of TH cells. Major TH cell subsets include TH1, TH2, and TH17, and also recently discovered TH9 and TFH cells. Each TH cell subset expresses a characteristic cytokine profile that generates a characteristic inflammatory response in allergic diseases, such as bronchial asthma. Among these TH cells, TH2 cells are believed to play a critical pathogenic role in allergic inflammation. TH2 cells produce cytokines, interleukin (IL)-4, IL-5, IL-6, IL-9, and IL-13, although they may also result from other cell types. Patients with allergic asthma have eosinophilic inflammation in the lung, in parallel with increased TH2 cytokines, as well as elevated serum IgE. IL-4 and IL-13 are representative TH2-type cytokines that play a crucial role in human allergic disease. IL-4 promotes differentiation and proliferation in TH2 cells, whereas IL-13 mediates AHR and mucus hyperproduction [1,2]. IL-5 is a cytokine that is highly specific to eosinophil activation and recruitment and contributes to eosinophilic inflammation, a prominent pathological feature in most asthma. TH2 cytokines also trigger the production of chemokines, including CCL11/eotaxin, CCL17/TARC, and CCL22/macrophage-derived chemokine (MDC), in tissue fibroblasts or epithelial cells, promoting the infiltration of inflammatory cells, such as eosinophils and TH2 cells into sites exposed to allergens. Importantly, IL-4 and IL-13 stimulate immunoglobulin class switching, leading to IgE production, which binds to its high-affinity receptor (Fc╬ĄRI) on the surface of mast cells or basophils. The association of captured allergens with IgE bound to Fc╬ĄRI on the cell surface activates signal transduction in these cells and rapidly leads to the release of inflammatory cytokines and chemical mediators, such as histamine and leukotrienes in mast cells and basophils and PGD2 in mast cells. Furthermore, particular interest has been generated in novel epithelial cell-derived cytokines including thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, which have all been implicated in promoting TH2 cytokine responses, and their ability to influence innate and adaptive immune responses associated with TH2 cytokine-mediated inflammation in airways. Recent evidence suggests that coordinated expression and cross-regulation between TSLP, IL-25, and IL-33 are crucial events for developing TH2 cell-mediated airway immune responses in asthma. In contrast, PGD2 modulates airway physiology by causing bronchoconstriction, vasodilation, increases in capillary permeability, and mucous production in asthma. These functions are well-known effects that may facilitate transendothelial migration of inflammatory cells, such as eosinophils, mast cells, lymphocytes, and monocytes, during allergic inflammation. In addition to such classical effects, novel properties of PGD2 include TH2 inflammation in allergic diseases (Fig. 1).
PGD2 and its metabolites
PG production begins with the liberation of arachidonic acid from membrane phospholipids by phospholipase A2 in response to inflammatory stimuli. Arachidonic acid is converted to PGH2 by the cyclooxygenase enzymes COX-1 and COX-2. PGH2 is a common precursor of several PGs, including PGD2, which is generated by the actions of two PG synthases, known as the lipocalin-type PGD synthase (L-PGDS), primarily expressed in brain, heart, and adipose tissue, and the hematopoietic PGD synthase (H-PGDS), mainly expressed in mast cells, macrophages, dendritic cells (DCs), and TH2 cells. It is generally thought that COX-1 is expressed constitutively in most tissues of the body and functions to maintain homeostatic processes, such as mucus secretion. In contrast, COX-2 is primarily an inducible enzyme, involved mainly in the regulation of inflammation [3]. PGD2 is a major product from COX-catalyzed reactions in a variety of tissues and cells, including those of the immune system, such as T cells, DCs, macrophages, mast cells, and platelets.
PGD2 production in asthma
Fujitani et al. [4] generated transgenic mice overexpressing lipocalin-like PGD synthetase in the lung and subjected them to ovalbumin (OVA)-induced pulmonary allergic inflammation. They found elevated IL-4 and IL-5 concentrations and increased eosinophilic infiltration in bronchoalveolar-lavage fluid (BALF) in these mice. Mandal et al. [5] reported that a reduction in PGD2 synthesis, induced by uteroglobin, an anti-inflammatory protein, was associated with reduced allergic inflammation. Furthermore, PGD2 nebulization before aerosol Ag challenge enhanced TH2 inflammatory responses, including eosinophilia, and leads to the development of AHR [6]. Collectively, these findings appear to substantiate the proposal that PGD2 acts as an important mediator in allergic asthma.
Activated mast cells contribute to asthmatic pulmonary inflammation by producing a variety of chemical mediators and cytokines. During allergic responses, PGD2 is released in large amounts by mast cells during asthmatic attacks in humans. DCs [7] and TH2 cells [8] also produce PGD2. Furthermore, fibroblasts, bronchial smooth muscle cells, and airway epithelial cells are also thought to produce PGD2, precipitating pulmonary inflammation. It is well established that the presence of an allergen triggers PGD2 production in sensitized individuals. In individuals with asthma, a bronchial allergen challenge leads to rapid PGD2 production, which can be detected in the BALF within minutes, reaching biologically active levels at least 150-fold higher than pre-allergen levels [9]. A local antigen challenge also stimulates PGD2 production in the nasal mucosa of patients with allergic rhinitis [10] and in the skin of patients with atopic dermatitis (AD) [11]. Several lines of evidence support the view that mast cells are the principal sources of PGD2 at allergic inflammation sites. Cell fractionation studies have shown that PGD2 is produced predominantly by mast cells [12], and mast cell activation is a requirement for PGD2 generation in chopped human lung parenchyma [13]. Thus, mast cells are responsible for the bulk of PGD2 production in the allergic setting. PGD2 produced by mast cells may provide an essential link between early-phase and late-phase allergic responses by initiating cellular processes that lead to the recruitment and activation of TH2 cells and eosinophils with associated pathophysiological effects.
PGD2-related receptors
Among PG receptors, D prostanoid receptor (DP)/DP1, DP2/chemoattractant receptor homologous molecule expressed on TH2 cells (CRTH2), and thromboxane A2 receptor (TP) belong to a family of seven-transmembrane G-protein-coupled receptors (GPCRs), mediating the biological effects of both PGD2 and its metabolites. In contrast, peroxisome proliferator-activated receptor (PPAR)-╬│ is a nuclear receptor for PGD2 metabolites. DP, TP, and PPAR-╬│ are present in various types of hematological and non-hematological cells [14,15]. In contrast, human CRTH2 has been specifically identified on hematological cells [16], although the tissue expression pattern of CRTH2 in humans differs from that in rodents [17].
DP is the most studied PGD2 receptor and its activation leads to Gs-mediated elevation in cAMP. DP is activated by PGD2 and its metabolites, including Δ12-PGD2 and PGJ2. Such activation by DP-selective agonists, such as BW245C, promotes relaxation of both vascular and airway smooth muscle, leading to vasodilatation and bronchodilatation, respectively [18]. DP is also expressed by platelets, in which its activation is linked to an anti-aggregatory function [19]. DP plays both anti-inflammatory and proinflammatory roles. Accumulation of cAMP is generally associated with the inhibition of effector cell function in lymphocytes, such as TH1 cells and natural killer cells, and other immune cells, [20]. Consistent with this, DP-mediated signals also suppress cell migration and/or the activation of eosinophils, basophils, netrophils, DCs, and fibroblasts [16,21-24]. Inhalation of a selective DP agonist suppresses asthma in a murine model by down-modulating lung DC function and inducing regulatory T cells. In contrast, DP is expressed by epithelial cells, fibroblast cells, and certain leukocyte populations [25-28] including monocytes, DCs, T cells, basophils, eosinophils, and mast cells, where it has been suggested to control several functions including the production of cytokines and lipid mediators. PGD2 synergizes with tumor necrosis factor via DP receptors to promote CCL2/MCP-1 and IL-8 production in monocytic cells [29]. urthermore, Matsuoka et al. [30] examined the role of DP in asthma by studying DP receptor-deficient mice in a model of OVA-induced allergic inflammation. Loss of DP did not affect the primary immune response, because similar serum total and OVA-specific IgE concentrations were observed in immunized wild-type and DP-deficient mice. However, the concentrations of TH2 cytokines, such as IL-4, IL-5, and IL-13, were significantly reduced, and lymphocyte and eosinophil accumulations in BALF were significantly suppressed in DP-deficient mice, compared with wild-type mice after the OVA challenge. While extensive cell infiltration and occasional bronchus-associated lymphoid tissue formation were consistently found in the lungs of all OVA-challenged wild-type mice, minimal cell infiltration was detected in the lungs of OVA-challenged DP-deficient mice. In contrast to wild-type mice, few mucus-containing cells were detected in the airways of DP-deficient mice. Moreover, whereas the OVA challenge significantly increased airway sensitivity to acetylcholine in wild-type mice, a minimal increase was detected in DP-deficient mice. Thus, PGD2 appears to act on DP and play an important role eliciting certain key features of allergic asthma. This has been confirmed by other experiments. Arimura et al. [31] administered the DP antagonist S-5751 orally to a similar guinea pig model of OVA-induced allergic asthma and found that it significantly alleviated eosinophil infiltration into the lung. Moreover, an association of DP promoter and gene polymorphisms with asthma has been detected in humans [32]. Thus, a DP-mediated PGD2 effect is expected to be involved in allergic and/or asthmatic responses.
CRTH2 was originally identified as an orphan receptor, expressed on type 2 polarized lymphocytes (TH2 and Tc2) [33], basophils, eosinophils [34], and monocytes [35], and mediates its effects by promoting the activation and chemotaxis of TH2 cells, eosinophils, basophils [16], and monocytes [35]. Despite binding to PGD2 with a similar affinity to DP, CRTH2 is not structurally related to DP and signals through a different mechanism. The effects of CRTH2 are mediated through a Gαi-dependent increase in intracellular calcium levels and a reduction in intracellular cAMP levels, indicating that CRTH2-mediated signals are predominantly proinflammatory. Unlike the prostanoid DP receptor, CRTH2 is a member of the chemoattractant receptor family, sharing higher sequence homology to the fMLP and C5a receptors than to the prostanoid receptor family [16]. The PGD2 metabolites, 13,14-dihydro-15-keto PGD2 (DK-PGD2), Δ12 PGD2, PGJ2, Δ12-PGJ2, 15d-PGJ2, and 9α, 11β-PGF2 bind to CRTH2 [16,17,36] and activate eosinophils [36-38]. PGF2α is a stereoisomer of 9α, 11β-PGF2, produced from PGH2 by the action of PGF synthase and from PGE2 by the action of PGE9-ketoreductase, independently of PGD synthetase. PGF2 is also implicated in CRTH2 signaling in the presence or absence of PGD2 production [39]. CRTH2 can also be activated by the thromboxane A2 metabolite, 11-dehydro-TBX2 [40]. The existence of these CRTH2 agonists suggests that this receptor may be of physiological importance even in the absence of basal PGD2 production. Ramatroban (BAYu3405) is an effective CRTH2 antagonist [41]. Ramatroban was originally identified as a TP antagonist, but it has been discovered that this drug binds to the CRTH2 receptor with moderate affinity (its antagonistic activity against CRTH2 is about 10-fold lower than that against TP) and blocks responses to the selective CRTH2 agonist DK-PGD2, in vitro and in vivo [41,42]. Uller et al. [43] studied the specificity of CRTH2 antagonism by TM30089, which is closely related structurally to dual TP/CRTH2 antagonists in mice. They assessed the inhibition of asthma-like pathological characteristics. Studies with these antagonists suggest that CRTH2 plays an important role mediating airway inflammation in response to an allergic challenge in both the guinea pig nasal mucosa [44] and mouse airway [45]. In humans, CRTH2 activation is responsible, at least in part, for the implications of PGD2 in asthma and inflammatory diseases [46,47] as well as allergic rhinitis [48] Thus, CRTH2 is considered to play an important role in allergic inflammation, similar to DP.
PGD2 enhances or suppresses inflammation by acting on different receptors expressed by hematopoietic and non-hematopoietic cells. Several cells of the immune system express both DP and CRTH2, which are coupled to apparently opposing signaling pathways. Because DP activation is often associated with inhibition of immune cell function [20], whereas CRTH2 activation leads to immune cell activation [20], it is tempting to hypothesize that these two PGD2 receptors collaborate to regulate inflammatory cell functions by different mechanisms. Although many immune cells coexpress DP and CRTH2, CRTH2-mediated signals often predominate over DP-mediated signals when cells are exposed to the non-selective agonist PGD2 [17,21,49], which binds to CRTH2 and DP with equal affinity [23,50]. One possible explanation for this observation may be the lower expression level of DP compared with that of CRTH2. Indeed, attempts to quantify DP and CRTH2 transcripts in immune cells, such as basophils, eosinophils, and TH2 cells, and in human airway smooth muscle cells have revealed significantly lower DP expression [4,21,24,28,51,52]. Because both DP and CRTH2 expression levels can be upregulated and downregulated by inflammatory stimuli [4,53,54], it is conceivable that the overall effect of PGD2 depends on the expression level of PGD2 receptors in a given cell.
Role of PGD2 in TH2 cell functions
TH cells, particularly those of the TH2 cell-related inflammatory response, have a crucial role in asthma pathogenesis. The TH2 type response is coordinated by TH2 cell differentiation and a modification of TH2 cell functions, such as cytokine production, recruitment, proliferation, survival, and apoptosis. Differentiation into each TH cell subset is delicately controlled in vivo by interactions with DCs. Airway DCs [25-27] express both CRTH2 and DP. The role of CRTH2 in DC function remains unclear, whereas DP-mediated regulation of DCs has been demonstrated in several studies. PGD2 suppresses the activation of DCs and prevents their migration into the T cell areas of draining lymph nodes [25]. This effect is mediated by DP. Indeed, it is mimicked by the DP-selective agonist BW 245C, but not by the CRTH2-selective agonist DK-PGD2 [25]. This suppressive mechanism by DP may underlie the inhibition of TH2 cell differentiation. Furthermore, inhaling a selective DP agonist suppresses the cardinal features of asthma by targeting the functions of lung DCs [55]. Interestingly, an increase in Foxp3+ CD4+ regulatory T cells was observed in mice treated with a DP agonist or DP-agonist-treated DCs, which suppressed inflammation in an IL-10-dependent manner. In contrast, it has been proposed that the DP-mediated inhibition of the production of TH1-inducing cytokines, such as IL-12 [26], by DCs favors T-cell development towards the TH2 phenotype [27]. This effect has been observed in preclinical models, such as TH1-dependent delayed-type hypersensitivity reactions [56], although additional mechanisms involving PPAR activation have been proposed in some cases.
Interestingly, DCs can themselves produce PGD2, which has been suggested to be involved in PGD2-mediated synthesis of CCL22/MDC, a chemoattractant for TH2 cells, in interferon (IFN)-╬│-treated human keratinocytes [57]. DCs not only function as target of PGD2, but also may play an important role in modulating local immunity and inflammation though self-producing PGD2.
Among human TH cell subsets, CRTH2 is preferentially expressed on TH2 cells, and is the most reliable marker to identify human CD4+ TH2 memory cells [32,58]. In contrast, DP is expressed on both TH1 and TH2 cells. Importantly, CRTH2 mediates the migration of TH2 cells towards PGD2 [16] and delays TH2 cell apoptosis. Recently, human CRTH2+ TH2 memory cells were reported to be maintained by TSLP-activated DC. Furthermore, CRTH2+CD4+ TH2 memory cells activated by TSLP-DCs undergo further TH2 polarization and express prostaglandin PGD2 synthase. Thus, in cooperation with DC-derived PGD2, TH2 cell-derived PGD2 may promote further accumulation of TH2 cells to the inflamed tissue [59] by positive feedback mechanisms, causing persistent inflammation and damage to the asthma airways.
Another notable role of PGD2 is regulating the production of proinflammatory cytokines in TH2 cells [28,51]. Tanaka et al. have shown that human CD4+ and CD8+ T cells producing IFN-╬│ and IL-2 are reduced in number by DP-mediated signals [28], whereas CRTH2-mediated signals enhance the ability of human TH2 cells to produce IL-2, IL-4, IL-5, and IL-13, an effect that was inhibited by ramatroban [51]. This PGD2-mediated effect on cytokine production by TH2 cells is mimicked by the selective CRTH2 agonist DK-PGD2 [51]. CRTH2-mediated PGD2 effects were dominantly observed on CRTH2+ TH2 cells, compared with DP-mediated effects after treatment with BW 245C, resulting in the attenuated production of cytokines by TH2 cells [28]. Furthermore, activation of TH2 cells in response to supernatants from immunologically activated human mast cells is mediated by PGD2, through an action on CRTH2 [60]. Together, these findings suggest that PGD2 favors TH2 functions through CRTH2 for recruiting TH2 cells to sites of allergic inflammation and for driving TH2-cytokine production, while restraining TH1 functions via DP, which may contribute to the development of TH2-dominated status during allergic inflammation. However, CRTH2 expression in mice is not biased towards TH2 cells, unlike humans [61], and the functions of CRTH2 may differ between mice and humans. Indeed, IL-4 and IFN-╬│ production was not affected in CRTH2-deficient mice. These results do suggest that the cytokines and chemokines regulated by CRTH2 differ between mice and humans. Furthermore, Chevalier et al. [62] found that CRTH2-deficient mice show enhanced eosinophil recruitment into the lung, compared with their wild-type littermates, and showed that CRTH2-deficient T cells produced significantly higher amounts of IL-5 and IL-3 in vitro. These results suggest a non-redundant role for CRTH2 in restricting eosinophilia and allergic responses in vivo. The authors suggested that one possible explanation for their findings was that CRTH2 promoted IL-5 production early on, and that the inhibitory pathway was subsequently turned on. In contrast, Satoh et al. [63] reported that allergic skin inflammation and IgE production were significantly diminished in CRTH2-deficient mice, consistent with the results of the pharmacological studies with human cells discussed above. Studies of allergic responses in mice in which CRTH2 has been genetically deleted have so far produced conflicting results. The CRTH2-mediated effects of PGD2 may interact in a complex fashion, with redundant roles and non-redundant roles, in stimulating cytokine production and allergic inflammation. These findings suggest that CRTH2 might be a highly promising therapeutic target, because CRTH2 blockade by small-molecule antagonists is likely to attenuate TH2 activity with respect to accumulation at sites of inflammation, cytokine release, and survival in tissue (Fig. 2).
Role of PGD2 in nonhematopoietic-cell functions
Although minimal expression of DP was detected in the lungs of non-immunized mice, an airway OVA challenge markedly enhanced DP receptor expression [30]. Immunoelectron microscopy, using an antibody to mouse DP, revealed that the DP receptor is highly expressed on ciliated and non-ciliated epithelial cells of the bronchioles and type II alveolar epithelial cells. Moderate expression was also found in type I alveolar epithelial cells and inflammatory white blood cells [30]. Airway epithelium has been proposed to be a source of proinflammatory cytokines and chemokines during asthma pathogenesis [64]. These findings suggest the following model for PGD2 in asthma: mast cell-derived PGD2 acts at DP receptors in the epithelium and stimulates the production and release of cytokines and chemokines to induce airway inflammation, obstruction, and hyper-reactivity. Chiba et al. reported that PGD2 induced IL-8 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells in association with mitogen-activated protein (MAP)/ERK and p38 MAP kinase activation via a CRTH2-independent pathway [65]. To further investigate the involvement of bronchial epithelial cells, Honda et al. [6] established a novel experimental model of asthma that permitted the direct assessment of the role of PGD2 in airway inflammation. Antigen-sensitized mice were exposed to aerosolized PGD2 1 day before challenge with low-dose aerosolized antigen. The results showed that not only the numbers of eosinophils, lymphocytes, and macrophages, but also the levels of IL-4 and IL-5 in BALF were higher in PGD2-pretreated mice than in control mice. Moreover, the expression of MDC, a TH2 cell chemoattractant, was higher in PGD2-pretreated mice than in controls. Furthermore, the authors showed that injecting anti-MDC antibody into PGD2-pretreated mice markedly inhibited inflammatory cell infiltration as well as TH2 cytokine production after antigen challenge. These results indicate that PGD2 accelerates TH2 type inflammation by inducing MDC. However, unfortunately, the PGD2 receptor required for the epithelial induction of MDC expression has yet to be identified, although DP is a candidate receptor for the initiation of MDC production in epithelial cells.
Recently, a novel aspect of DP in airway epithelial cells was shown by Mandal et al. [5]. They demonstrated that uteroglobin (UG)-deficient mice had elevated levels of PGD2 in BALF and increased COX-2 gene expression in epithelial cells. They also found that DP signaling was mediated via p38 MAP kinase (MAPK), p44/42 MAPK, and protein kinase C (PKC) pathways in a cell type-specific manner, leading to the activation of nuclear factor (NF)-╬║B, which then stimulated COX-2 expression.UG as also found to bind PGD2, block DP signaling, inhibit NF-╬║B activation, and, consequently, suppress COX-2 gene expression. These studies demonstrated that DP signaling mediated allergic inflammatory responses by activating NF-╬║B, which stimulated COX-2 expression. This phenomenon is also observed in fibroblasts and smooth muscle cells. Thus, in asthma, DP augments the production of COX-2 metabolites of arachidonic acid, including PGD2, in airway epithelial cells by an autocrine mechanism, resulting in their local accumulation and further production of chemokines. UG thus appears to be a key component of an innate homeostatic mechanism, acting to prevent stimulation of an allergen-induced, DP-mediated inflammatory response (Fig. 2).
Role of PGD2 in inflammatory cell functions (mast cells, eosinophils, and basophils)
TH2 cells play an important role in allergic disease by organizing the characteristic inflammatory response, involving mast cells, basophils, and eosinophils. These inflammatory cells also produce TH2 type cytokines, indicating that these cells and TH2 cells interact to promote TH2 type inflammation in asthma. While the above studies suggest an important role for PGD2 in asthma pathogenesis, DP suppresses mast cell activation. Chan et al. [66] collected rat peritoneal mast cells and examined the effects on anti-IgE-induced histamine release of agonists specific to various types of prostanoid receptors. They found that several prostanoid agonists inhibited histamine release; BW 245C and ZK 118182 were the most potent. Based on these findings, they suggested that developing highly specific agonists for the mast cell DP receptor might improve the management of allergic diseases, such as asthma. In contrast, Matsuoka et al. [30] showed that the net action of DP in allergic asthma appears to be facilitative, given that DP is present. Strong evidence supporting a role for CRTH2 in mast cells is lacking, although the receptor is expressed. In contrast, activating CRTH2 induces migration of murine mast cells, based on the upregulation of CD23 and CD30 on the cell surface.
Eosinophils express both DP and CRTH2, which interact to regulate cellular responses to PGD2. Previous studies have revealed that CRTH2 mediates eosinophil chemotaxis induced by mast cell products [34]. The receptor directly responsible for stimulating eosinophil migration appears to be CRTH2, not DP [16]. Gervais et al. [21] found that DK PGD2, a CRTH2 agonist, stimulated chemokinesis and degranulation of eosinophils, whereas BW 245C did not. CRTH2 activation also leads to changes in the shape of eosinophils, chemotaxis, enhanced chemotactic responsiveness to other chemoattractants, and degranulation [21,22,38,39], whereas DP activation is linked to eosinophil apoptosis [21], hich is delayed by BW 245C but not DK-PGD2. However, inhibiting DP with the selective antagonist BW A868C resulted in markedly enhanced CRTH2-mediated activation, as assessed by an increase in CD11b expression on exposure to PGD2 [22]. Novel effects of PGD2 on eosinophils have been reported by Mesquita-Santos et al. [67]. They showed that administering PGD2 to mice enhanced leukotrienes (LT) C4 production by inducing lipid body-driven LTC4 synthesis, dependant on the synergistic activity of endogenous eotaxin, acting via the CCR3 receptor.
Opposing effects of DP and CRTH2 on cell function have also been observed in basophils, circulating leukocytes that are particularly relevant during the late phase of the allergic response and for asthmatic inflammation [24]. CRTH2 signaling is responsible for mobilizing intracellular Ca2+ in basophils, upregulating CD11b and CD203c expression, and enhancing IgE-mediated basophil degranulation [68]. Conversely, DP activation inhibits basophil migration and IgE-mediated degranulation. Thus, it seems that activating basophils and eosinophils, balanced by PGD2 via the dual receptor system, plays an important role in asthma.
According to CRTH2 and DP studies, these two receptor subtypes may collaborate in the activation and accumulation of inflammatory cells, such as mast cells, eosinophils, and basophils, at sites of allergic inflammation (Fig. 2).
Role of the PGD2 receptor in allergic disease pathogenesis
Recent studies have shown an accumulation of CRTH2-positive leukocytes, including TH2 cells, eosinophils, basophils, and mast cells, in human allergic inflammatory regions. Furthermore, CRTH2 mRNA and protein levels are higher in eosinophils from atopic subjects than in those from healthy controls [69]. Consistent with the increased number of CRTH2 receptors in eosinophils from atopic individuals, PGD2-induced chemotaxis is significantly higher in these cells. Increased levels of CRTH2 expression and an enhanced sensitivity of eosinophils to PGD2 might explain the tissue eosinophilia in atopic patients. Furthermore, the percentage of CD4+ T cells expressing CRTH2 in patients with AD correlates with disease severity [70-72]. Additionally, available evidence indicates that genetic alterations in CRTH2 are related to allergic asthma. Genetic variants of CRTH2 predispose individuals to develop asthma. CRTH2 is associated with severe asthma in African-American and Chinese populations [70]. Associated polymorphisms in the 3'-untranslated region of CRTH2 lead to increased mRNA stability, suggesting that CRTH2 variants are associated with higher degrees of bronchial hyperresponsiveness and are causally linked to asthma [70]. These findings indicate that higher CRTH2 expression levels and increased responsiveness to its PGD2 ligand may be the biological basis for the association of this receptor with asthma. In contrast, variants of PTGDR (the gene encoding DP1) with reduced transcriptional activity have been detected in patients with asthma [73]. Studies in a small cohort of patients with asthma provided evidence that these putative loss-of-function variants protect against asthma development.
CONCLUSION
PGD2 has been recognized as an important mediator of allergic responses. It is becoming clear that PGD2 and its metabolites may play important roles in the pathogenesis of allergic diseases, including asthma. Although the potential clinical benefits of modifying PGD2-related receptor function remain unsubstantiated, many studies have provided promising evidence that these receptors are immune and inflammatory regulators in various immune cells. The roles of DP and CRTH2 are diverse and complex in the allergic system. Thus, future studies into the function (s) of these receptors will likely lead to new approaches for the treatment and, perhaps, prevention of allergic diseases, including asthma.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





