 |
 |
| Korean J Intern Med > Volume 24(4); 2009 > Article |
|
Abstract
Nonmyeloablative stem cell transplantation (NST) is increasingly used with beneficial effects because it can be applied to older patients with hematological malignancies and those with various complications who are not suitable for conventional myeloablative stem cell transplantation (CST). Various conditioning regimens differ in their myeloablative and immunosuppressive intensity. Regardless of the type of conditioning regimen, graft-versus- host disease (GVHD) in NST occurs almost equally in CST, although a slightly delayed development of acute GVHD is observed in NST. Although graft-versus-hematological malignancy effects (i.e., graft-versus-leukemia effect, graft-versus-lymphoma effect, and graft-versus-myeloma effect) also occur in NST, completely eradicating residual malignant cells through allogeneic immune responses is insufficient in cases with rapidly growing disease or uncontrolled progressive disease. Donor lymphocyte infusion (DLI) is sometimes combined to support engraftment and to augment the graft-versus-hematological malignancy effect, such as the graft-versus-leukemia effect. DLI is especially effective for controlling relapse in the chronic phase of chronic myelogenous leukemia, but not so effective against other diseases. Indeed, NST is a beneficial procedure for expanding the opportunity of allogeneic hematopoietic stem cell transplantation to many patients with hematological malignancies. However, a more sophisticated improvement in separating graft-versus-hematological malignancy effects from GVHD is required in the future.
Myeloablative conditioning regimens with high-dose chemotherapy, with or without a lethal dose of total body irradiation, have been applied to eradicate underlying disease and suppress the host's immune system to achieve engraftment and disease control in allogeneic hematopoietic stem cell transplantation (HSCT). This type of HSCT causes profound marrow suppression and organ toxicity. Recently, nonmyeloablative stem cell transplantation (NST) has been widely and increasingly used in clinical allogeneic HSCT, based on findings that engraftment can also be succeeded by nonmyeloablative conditioning regimens, which are mainly composed of immune suppression. The term reduced-intensity allogeneic hematopoietic stem cell transplnantation (RIST) is also used for any transplantation that uses a conditioning regimen other than myeloablative. Although a RIST regimen that is of minimal intensity is called NST in a strict sense, the term NST is synonymously used with RIST in the present review. Originally, NST was developed by several researchers about 10 years ago [1-7]. NST is beneficial for older patients (generally over 50-55 years old) and those with comorbidities because nonmyeloablative conditioning regimens are less toxic for the bone marrow as well as the other organs and tissues, resulting in reduced transplant-related mortality (TRM). Other major complication such as graft-versus-host disease (GVHD) usually occur when a conversion occurs from mixed chimerism to complete donor chimerism, which is accompanied by the graft-versus-leukemia (GVL) effect. To obtain complete donor chimerism, donorlymphocyte infusion (DLI) is the usual procedure that supports enhanced engraftment and suppresses the host-versus-graft reaction, and sometimes induces the GVL effect [8-14]. However, similar to allogeneic myeloablative stem cell transplantation, separation of the GVL effect from GVHD is difficult.
Allogeneic immune responses can be induced against other hematological malignancies. An anti-lymphoma or anti-myeloma effect is also observed after allogenic HSCT and is called the graft-versus-lymphoma effect or graft-versus-myeloma effect, respectively. The term GVL effect is used here to include representatives of graft-versus-hematological malignancy effects.
Although NST has been applied to solid tumors such as renal cell carcinoma, breast cancer, and melanoma with a great expectation for a cure, a marked antitumor effect (graft-versus-tumor effect) often accompanies severe GVHD [15,16]. Thus, NST for solid tumors is now being performed only in a limited number of institutions.
Based on the frequent coexistence of a GVL effect with GVHD in NST and conventional myeloablative stem cell transplantation (CST), efficient separation of the GVL effect from GVHD is eagerly anticipated. The separation of the phenomena appears to be realized by progress in transplantation immunology, utilization of minor histocompatibility antigen expression differences between donor and host, tumor-associated antigens for vaccination or cytotoxic T-cell induction, cellular therapies, and new pharmacologic agents. These strategies are beneficial not only for NST but also for CST to enhance the GVL effect without inducing severe GVHD.
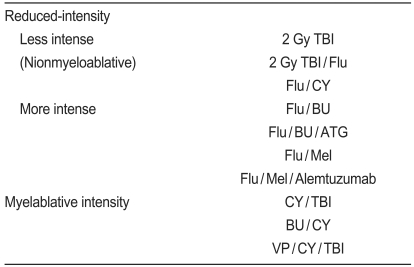
NST was developed by several investigators with various conditioning regimens that are different from conventional myeloablative HSCT (Table 1). The representative CST is as follows: cyclophosphamide (CY) 60 mg/kg├Ś2 days+ total body irradiation (TBI) 2 Gy├Ś12, busulfan (BU) 1 mg/kg├Ś16+CY 50 mg/kg├Ś4 days, and VP-16 10 mg/kg├Ś2 days+CY 60 mg/kg├Ś2 days+TBI 2 Gy├Ś6. In contrast, NST regimens have reduced-intensity compared to those of CST. Each regimen shows somewhat different GVHD incidences and GVL effects. The M.D. Anderson Cancer Center and the UK groups [16-18] use fludarabine (Flu) 30 mg/m2/day├Ś4-5 days+melphalan (Mel) 140 mg/m2/day├Ś1 day┬▒alemtuzumab 20 mg/day├Ś4-5 days. The Hadassah University Hospital group and others [2,19,20] use Flu 30 mg/m2/day├Ś6 days or cladribine 12 mg/m2/day├Ś5 days+BU 4 mg/m2/day├Ś2 days┬▒anti-T-lymphocyte globulin (ATG) 10 mg/kg/day├Ś4 days. The Massachusetts General Hospital group uses Flu 30 mg/m2/day+CY 50 mg/kg/day├Ś4 days+ATG 15 or 30 mg/kg/day├Ś2 days+7 Gy thymic irradiation. The NIH and M.D. Anderson Cancer Center groups [5,15,21,22] use Flu/CY.
The Fred Hutchinson Cancer Research Center group [3,23,24] uses 2 Gy TBI┬▒Flu 30 mg/m2/day├Ś3 days. Among reduced-intensity conditioning regimens, Flu/Mel┬▒alemtuzumab and Flu/BU┬▒ATG are relatively close to the myeloablative conditioning regimens. In contrast, Flu/CY and TBI 2 Gy┬▒Flu are milder conditioning regimens than Flu/BU┬▒ATG and Flu/Mel. Therefore, DLI is often needed to obtain complete donor chimerism in cases with the most reduced-intensity conditioning regimens such as Flu/CY and TBI 2 Gy┬▒Flu. The conditioning regimen intensity correlates with antileukemic activity, regimen-related toxicity, and time to achieve complete donor chimerism. Further descriptions about the differences in each procedure can be found elsewhere. Delineating the incidence of GVHD and GVL effects is more important in NST as well as in CST and it enhances the GVL effect without augmenting GVHD.
NST induces mixed chimerism, followed by a decreased early onset of grades II to IV (mild to severe) acute GVHD. However, late-onset (>100 days after NST) acute GVHD and chronic GVHD occur similarly to those in CST. Initially, the incidence and severity of acute GVHD was thought to be less in NST than in CST because NST results in milder tissue toxicity and does not induce a cytokine storm, which is one of the major inducers of acute GVHD and possibly chronic GVHD [25-28]. Although the incidence and severity of acute GVHD is somewhat delayed in NST compared to CST, it is not much different from those of CST. The only difference is the delayed occurrence of acute GVHD in NST as compared to CST and it partly depends on the delayed establishment of complete donor chimerism and utilization of DLI to inhibit rejection, promote engraftment, and enhance the GVL effect. The DLI dose is not always correlated with GVHD and the GVL effect and unfortunately both phenomena often coexist.
One of the advantages of NST is less toxicity against various organs and tissues; thus, it is applicable to older patients and those with limited organ dysfunction. This is a great advantage for NST because now many patients can receive allogeneic HSCT in a NST fashion. Another advantage is the expected recovery of autologous hematopoiesis, even if engraftment failure occurs; however, this characteristic is correlated with frequent mixed chimerism and relapse of leukemias or other hematological malignancies.
Several studies have shown that the outcomes of older patients who undergo NST during remission are comparable to those of patients who receive CST [29-31], suggesting that the GVL effect associated with NST might be adequate for controlling chemosensitive or slowly progressing disease. Although whether NST is feasible for patients not in remission is controversial [32-35], Maruyama et al. [36] showed that the GVL effect associated with NST is comparable to that associated with CST.
Clinical outcome in various hematological malignancies after NST varies from disease to disease and depends on disease status and its growing capacity (Table 2).
Regardless of the various conditioning regimens, the occurrence of acute GVHD (Ōēźgrade II), chronic GVHD, 2-year overall survival (OS), and NST relapse rate are about 35%, 40%, 40%, and 50%, respectively, suggesting similar outcomes to those in CST [37,38]. However, NST is superior to chemotherapy in patients older than 50 years with AML and in those in their first complete remission [39]. An additional randomized trial is needed to determine whether NST in younger patients with AML/MDS is also superior to CST.
The Israeli group showed that the 5-year disease-free survival rate for chronic phase CML (CML-CP) is about 85% [40], whereas the European Group for Blood and Marrow Transplantation showed a 5-year OS of about 60% in CML-CP and about a 20% 5-year OS in accelerated phase or blastic crisis CML (CML-AP/BC) [41]. Since new drugs such as imatinib, nilotinib, and dasatinib have been developed and are extensively effective CML-CP agents, the frequency of allogeneic HSCT including NST is decreasing, and allogeneic HSCT is performed only in cases of advanced-phase CML. More intense conditioning regimens rather than minimally reduced conditioning regimens are recommended when NST is chosen for CML [42].
Generally, the GVL effect after allogeneic HSCT is hardly generated in ALL, but some GVL effect may occur [43-45]. Furthermore, the GVL effect for ALL might be supported by the result of a retrospective analysis in Japan, which showed that allogeneic HSCT for ALL was superior to autologous HSCT and dissimilar to AML [46]. Another aspect of allogeneic HSCT for ALL is to intensify the conditioning regimen, although NST has more often been performed for various hematological diseases. In fact, an excellent outcome (about a 90% 3-year OS) was observed in allogeneic HSCT using a conditioning regimen with medium-dose VP-16, cyclophosphamide, and total body irradiation for patients with ALL in their first complete remission [47]. NST is not often performed for such patients to justify its efficacy for the GVL effect. In general, because a slight GVL effect may be induced even by the CST setting, NST is likely inadequate for ALL.
CLL is an incurable disease with standard chemotherapy. Although autologous HSCT shows little evidence of a beneficial outcome, allogeneic HSCT with myeloablative conditioning regimens have established high and complete response rates but often with high TRM [48]. The presence of a GVL effect in CLL is evident in the induction of complete remission by DLI [48,49]. Therefore, NST has been applied to CLL as well. TRM is reduced by NST, but this benefit is offset by increased relapse rates [50].
Autologous HSCT is usually performed in patients with NHL, and allogeneic HSCT is applied to relapsed, chemoresistant, or poor-prognosis patients. The outcome of NST for high-grade NHL varies widely [22,51,52]. In contrast, low-grade, advanced-phase NHLs, especially follicular lymphoma, are well treated with NST, resulting in about an 80% 3-year OS [53].
Allogenic HSCT with myeloablative conditioning regimens is too toxic to patients with multiple myeloma and results in a higher TRM [54], although a graft-versus-myeloma effect does exist [55,56]. Single or double autologous HSCT is often used for multiple myeloma treatment, but controversial results have been reported [57,58]. NST following autologous HSCT has also been examined and results in an excellent outcome [59,60]. However, controversy still exists concerning the outcome between double autologous HSCT and autologous HSCT followed by NST [61]. Furthermore, better response rates are obtained using various new drugs such as tahidomide, lenalidomide, and bortezomib in combination with dexamethasone, prednisolone, and/or melphalan, and when using those as maintenance therapeutic drugs after single autologous HSCT [62-64]. So, the efficacy of NST for multiple myeloma has not yet been confirmed.
DLI is often required to achieve complete donor chimerism in minimally reduced-intensity conditioning regimens. However, it is not so frequently required to achieve complete donor chimerism after moderately reduced-intensity conditioning regimens, which are similar to myeloablative conditioning regimens; therefore, it is currently used only for relapse or after an increase in host cells during a period of mixed chimerism. Table 2 summarizes the anti-hematological malignancy effects by DLI in various hematological diseases.
Initially, DLI was reported to be markedly effective against CML relapse after allogeneic HSCT. About 80% of patients treated with DLI for relapsed CML-CP will achieve a complete cytogenetic and molecular response [14,65]. In contrast, only 12-28% of patients with accelerated phase or blast crisis relapse respond to DLI. Advanced-phase relapse rates after DLI are high [66,67]. Although DLI results in an excellent GVL effect against relapsed CML, its effect is disappointing inrelapsed AML, exhibiting about 20-30% complete remission [14,65]. Furthermore, DLI is less effective for managing posttransplant MDS. The GVL effect of DLI is not remarkable in cases of ALL, showing only about a 10% complete remission [65]. The response rate for DLI in patients with multiple myeloma is about 30%, but a sustained remission occurs in 0-18% of patients [55,68-70]. In addition to the limited response rate, DLI toxicity is significant for myeloma, with more than half of patients experiencing acute and chronic GVHD. Therefore, a graft-versus-myeloma effect has been implicated in other settings such as RIST or the prophylactic use of DLI following T-cell-depleted HSCT. [71,72]. Furthermore, new drugs such as bortezomib and thalidomide are often used for myeloma in combination with other drugs, so the advantage of allogeneic HSCT for the treatment of multiple myeloma is being reconsidered. Although Hodgkin's lymphoma (HL) and NHL are susceptible to the DLI graft-versus-lymphoma effect to some extent [52,73-75], the GVL effect appears to be readily generated in the NST setting for HL and low-grade NHL [53,76]. Acute GVHD develops in 19-33% of DLI after NST, and chronic GVHD occurs in 33-34%. No statistically significant relationship exists between GVHD and DLI dose in some occasion [77,78].
DLI is performed to avoid graft rejection or malignancy relapse and is marginally effective for controlling such episodes, although not always. A chimerism analysis, which shows the proportion of donor-derived and host-derived cells, is important to determine the appropriate timing for DLI. Several methods can be used to analyze chimerism. Among them, using microsatellite DNA as the RT-PCR probe is useful in terms of accuracy and quantity [79]. Although donor T-cell chimerism is believed to be a good indicator for estimating engraftment and rejection, donor NK-cell chimerism at the early phase after transplantation is also a valuable indicator [79,80]. BecauseNK cells recover faster than T cells, donor NK-cell chimerism predicts subsequent donor T-cell chimerism and engraftment. Murine models suggest the importance of host NK cells for rejection of allogeneic hematopoietic stem cells [81-83]. In fact, more frequent rejection occurs when donor CD56+ NK-cell chimerism is less than 50% on day 14, resulting in an unfavorable outcome [79]. Grade II to IV acute GVHD develops in patients with more than 50% donor-type chimerism in CD3+ T cells on day 14. Furthermore, more than 50% of donor-type chimerism in CD56+ NK cells on day 14 and more than 75% donor-type chimerism in CD3+ T cells, CD56+ NK cells, and CD14/15+ myeloid cells on day 28 are associated with a lower relapse rate. Better 1-year overall survival is shown in patients with more than 50% donor-type chimerism in CD56+ NK cells on day 14 and more than 90% donor-type chimerism in CD14/15+ myeloid cells and CD56+ NK cells on day 28.
Regarding the GVL effect, Baron et al. [84] reported that extensive chronic GVHD, but not acute GVHD, is associated with a decreased risk of relapse or progression and an increased probability of progression-free survival. Although achievement of complete donor chimerism was associated with a decreased risk for relapse or progression, grade II to IV acute GVHD had no significant impact on the risk of relapse or progression but was associated with an increased risk for non-relapse mortality and decreased probability of progression-free survival. The reason why acute GVHD was not associated with an increased probability of achieving complete remission in their study appeared to be that corticosteroids and other immunosuppressive agents used to treat acute GVHD lowered the GVL effect.
In any case, a chimersim analysis on various cell lineages, such as CD3+ T cells, CD56+ NK cells, and CD14/15+ myeloid cells, is important to estimate the appropriate timing of DLI and to evaluate the occurrence of rejection, relapse, and GVHD in NST.
The presence of host-derived APCs during the first month after NST may be responsible for efficient donor T-cell immunization against host hematopoietic cells. Strong antileukemic responses are seen in some patients without clinical GVHD, suggesting that those responses were directed against antigens preferentially expressed on hematopoietic cells, although this situation cannot exclude the existence of a subclinical level of graft-versus-host reaction (GVHR). In general, achievement of complete donor T-cell chimerism is associated with a reduced risk of relapse or progression, indicating that alloreactivity against both normal host hematopoiesis (GVHR or GVHD) and leukemic cells (GVL effect) were effectively induced.
However, complete donor chimerism increases non-relapse mortality. This observation can cause the apparent strong association between high levels of donor T-cell chimerism early after NST and the increased risk of more severe GVHD. Therefore, a transient mixed chimerism after NST appears to be requisite to induce the GVL effect, and possibly GVHR/GVHD as well, through donor T-cell immunization by host APCs. In fact, the absence of host APCs by day 100 after NST leads to reduced acute GVHD [85], whereas the GVL effect can be induced by crosspresentation in which donor T cells are primed with host leukemia antigens presented by donor APCs [86]. Host APCs, however, have recently been suggested as being necessary to induce both the GVHD and GVL effect; moreover, APCs were also found to be necessary to induce both the GVHD and GVL effect, showing the absence of a clear association between donor/host APCs and the GVHD/GVL effect. Although Matte et al. [87] reported that GVHD is intensified by donor APCs cross-priming alloreactive CD8+ T cells, and that donor APCs are not required for the CD8-mediated GVL effect, Reddy et al. [88] showed that APCs and alloantigen tumor expression are crucial for the GVL effect, and that host APCs predominate in the GVL effect with the contribution of donor APCs to decrease tumor burden. Furthermore, donor or host APCs are associated with CD4-mediated chronic skin GVHD via CD80/CD86 dependent costimulation, and both donor and host APCs elicit maximal chronic GVHD, whereas donor APCs play a dominant role in CD4-mediated intestinal chronic GVHD via CD40 and CD80/CD86-dependent costimulation [89]. Because host APCs are required to initiate CD8-mediated acute GVHD [90,91], differences in APC requirements exist between CD8-mediated acute GVHD and CD4-mediated chronic GVHD, with target tissue-specific differences as well.
The separation of the GVL effect from GVHD is one of the major issues in allogeneic HSCT. Various trials have been conducted to examine such conditions in murine models. For example, utilization of a cytokine balance toward Th1/Tc1 may preferentially lead to the GVL effect. A special T-cell subset expressing CD62L- CD4+ effector memory T cells appears to play an important role for exerting the GVL effect without augmenting GVHD. Utilization of alloreactive NK cells can deplete host APCs, resulting in reduced GVHD with a preserving GVL effect. Blockade of costimulatory molecules such as CTLA4-Ig, anti-OX40L antibody, and anti-ICOS antibody may inhibit GVHD and preserve the GVL effect [92-98]. Chemokine and chemokine receptor modulation by the relevant antibody or antagonist may also inhibit GVHD, but not the GVL effect [99-108]. Novel pharmacologic agents such as the proteasome inhibitor bortezomib plus allogeneic T-cell infusion induce the GVL effect without enhancing GVHD [109]. Furthermore, the histone deacetylase inhibitor suberoylanilide hydroxamic acid induces the GVL effect, but not GVHD [110].
In humans, alloreactive NK cells can inhibit GVHD and preserve the GVL effect, so in vivo expansion after allogeneic HSCT and transfer of in vitro-generated alloreactive NK cells appear to inhibit GVHD, but not GVL or the graft-versus-tumor effect [91,111-113]. Rapamaycin appears to induce the GVL effect, inhibiting GVHD in humans [114,115]. Recently, CCL8 has been shown to be a molecular candidate for the diagnosis of acute GVHD in both mice and humans [116,117], and a four protein fingerprint of interleukin-2 receptor-╬▒, tumor necrosis factor receptor-1, interleukin-8, and hepatocyte growth factor in plasma have been identified as biomarkers for predicting severe acute GVHD [118]. Therefore, determining whether blocking of these molecules inhibits GVHD and preserves the GVL effect would be of interest.
Differences in minor histocompatibility antigen expression between donor and host can be utilized to separate the GVL effect from GVHD, although a limitation exists in exploiting such disparities in terms of difficulty in HLA-matched donor selection and HLA-restricted immune response of anti-minor histocompatibility antigens [119-121]. HA-2 is preferentially expressed on hematopoietic tissue rather than nonhematopoietic tissue; therefore, anti-host HA-2-specific T cells induce the GVL effect without augmenting GVHD. In contrast, HA-1 is expressed on both hematopoietic and non-hematopoietic tissues, and HA-3 is preferentially expressed on nonhematopoietic tissues, suggesting that these minor histocompatibility antigens are not suitable to induce only the GVL effect. The Y chromosome encodes male-specific minor histocompatibility (HY) antigens such as UTY, SMCY, and DBY; therefore, anti-HY-specific T cells can be induced after female to male allogeneic HSCT [122-124]. Because only UTY is modestly expressed on host epithelial cells, anti-UTY-specific T cells may lead to the GVL effect without augmenting GVHD. In contrast, SMCY and DBY are highly expressed on epithelial cells, thus causing anti-SMCY- and DBY-specific T cells that appear to induce both GVHD and the GVL effect. Furthermore, DDX3Y, which is expressed on leukemic stem cells, appears to be a suitable target for the GVL effect, but not for GVHD, because DDX3Y expression is detected in all myeloid and lymphoid leukemic cells that carry an intact Y chromosome [125].
Other candidate antigens that exert only the GVL effect, but not GVHD, are as follows: proteinase 3, which is aberrantly expressed or overexpressed in AML and CML [126-128]; WT-1 in AML, CML, and multiple myeloma [129-132]; survivin for AML, CML, CLL, and lymphoma [133-136]; idiotype for multiple myeloma [137]; and BCR-ABL [138] for CML. In vitro-generated cytotoxic T cells against various minor histocompatibility antigens can be used for adoptive transfer, and peptide vaccine trials for BCR-ABL, PR1 derived from PR3, and WT1 are being examined. The efficacy of the BCR-ABL vaccine is limited in patients with low levels of residual and stable disease. In contrast, the PR1 and WT1 vaccines are more beneficial, resulting in better clinical responses [139]. However, vaccination therapy with only these peptides is not enough to obtain a sufficient clinical outcome. Recently, unique HLA-mismatch combinations between donor and host HLA-C expression have been correlated with the induction of an efficient GVL effect without augmenting GVHD by a retrospective analysis using data from the Japan Marrow Donor Program [140,141]. This type of approach is one of the candidates for separating the GVL effect from GVHD, although great genetic differences exist among nations regarding GVHD and HLA disparities.
The efficacy and limitations of NST are becoming clearer because this procedure alone cannot sufficiently induce the graft-versus-hematological malignancy effect except for certain diseases, even in combination with DLI. Therefore, future progress in transplantation immunology is needed, so that we are able to efficiently separate graft-versus-hematological malignancy from GVHD. Furthermore, NST should be combined with various strategies such as the utilization of minor histocompatibility antigen expression differences, vaccination, or induction of cytotoxic T cells using tumor-associated antigens and other cellular therapies to enhance the graft-versus-hematological malignancy effect without augmenting GVHD.
References
1. Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood 1997;89:4531ŌĆō4536PMID : 9192777.


2. Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998;91:756ŌĆō763PMID : 9446633.


3. McSweeney PA, Niederweiser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001;97:3390ŌĆō3400PMID : 11369628.


4. Spitzer TR, McAfee S, Sackstein R, et al. Intentional induction of mixed chimerism and achievement of antitumor responses after nonmyeloablative conditioning therapy and HLA-matched donor bone marrow transplantation for refractory hematologic malignancies. Biol Blood Marrow Transplant 2000;6:309ŌĆō320PMID : 10905768.


5. Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem celltransplantation: full donor T-cell chimerism precedes alloimmune responses. Blood 1999;94:3234ŌĆō3241PMID : 10556212.


6. Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 2000;96:2419ŌĆō2425PMID : 11001893.


7. Bornhauser M, Thiede C, Platzbecker U, et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res 2001;7:2254ŌĆō2262PMID : 11489799.

8. Storb R, Doney KC, Thomas ED, et al. Marrow transplantation with or without donor buffy coat cells for 65 transfused aplastic anemia patients. Blood 1982;59:236ŌĆō246PMID : 7034811.



9. Sullivan KM, Storb R, Buckner CD, et al. Graft-versus-host disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. N Engl J Med 1989;320:828ŌĆō834PMID : 2648143.


10. Kolb H, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990;76:2462ŌĆō2465PMID : 2265242.


11. Porter DL, Roth MS, McGarigle C, et al. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med 1994;330:100ŌĆō106PMID : 8259165.


12. Mackinnon S, Papadopoulos EB, Carabasi MH, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood 1995;86:1261ŌĆō1268PMID : 7632930.


13. Andreani M, Manna M, Lucarelli G, et al. Persistence of mixed chimerism in patients transplanted for the treatment of thalassemia. Blood 1996;87:3494ŌĆō3499PMID : 8605369.


14. Collins RH Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 1997;15:433ŌĆō444PMID : 9053463.


15. Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal cell carcinoma after nonmyeloablative allogeneic peripheral blood stem-cell transplantation. N Engl J Med 2000;343:750ŌĆō758PMID : 10984562.


16. Ueno NT, Rondon G, Mirza NQ, et al. Allogeneic peripheral-blood progenitor-cell transplantation for poor-risk patients with metastatic breast cancer. J Clin Oncol 1998;16:986ŌĆō993PMID : 9508181.


17. Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 2001;97:631ŌĆō637PMID : 11157478.


18. Chakraverty R, Peggs K, Chopra R, et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantation by using a nonmyeloablative conditioning regimen. Blood 2002;99:1071ŌĆō1078PMID : 11807015.


19. Bornhauser M, Thiede C, Schuler U, et al. Dose-reduced conditioning for allogeneic blood stem cell transplantation: durable engraftment without antitymocyte globulin. Bone Marrow Transplant 2000;26:119ŌĆō125PMID : 10918420.


20. Saito T, Kanda Y, Kami M, et al. Therapeutic potential of a reduced-intensity preparative regimen for allogeneic transplantation with cladribine, busulfan, and antithymocyte globulin against advanced/refractory acute leukemia/lymphoma. Clin Cancer Res 2002;8:1014ŌĆō1020PMID : 11948108.
21. Khouri IF, Keating M, Korbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 1998;16:2817ŌĆō2824PMID : 9704734.


22. Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle cell lymphoma. J Clin Oncol 2003;21:4407ŌĆō4412PMID : 14645431.


23. Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood 2003;102:2021ŌĆō2030PMID : 12791654.


24. Feinstein LC, Sandmaier BM, Hegenbart U, et al. Nonmyeloablative allografting from human leukocyte antigen-identical sibling donors for treatment of acute myeloid leukaemia in first complete remission. Br J Haematol 2003;120:281ŌĆō288PMID : 12542488.


25. Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood 1992;80:2964ŌĆō2968PMID : 1467511.


26. Tanaka J, Imamura M, Kasai M, et al. Cytokine gene expression in peripheral blood mononuclear cells during graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol 1993;85:558ŌĆō565PMID : 8136279.


27. Imamura M, Hashino S, Kobayashi H, et al. Serum cytokine levels in bone marrow transplantation: synergistic interaction of interleukin-6, interferon-╬│, and tumor necrosis factor-╬▒ in graft-versus-host disease. Bone Marrow Transplant 1994;13:745ŌĆō751PMID : 7920309.

28. Tanaka J, Imamura M, Kasai M, et al. The important balance between cytokines derived from type-1 and type-2 helper T cells in the control of graft-versus-host disease. Bone Marrow Transplant 1997;19:571ŌĆō576PMID : 9085737.


29. Alyea EP, Kim HT, Ho V, et al. Comparative outcome of nonmyeloablative and myeloabltaive allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood 2005;105:1810ŌĆō1814PMID : 15459007.


30. Valcarcel D, Martino R, Sureda A, et al. Conventional versus reduced-intensity conditioning regimen for allogeneic stem cell transplantation in patients with hematological malignancies. Eur J Haematol 2005;74:144ŌĆō151PMID : 15654906.


31. Scott BL, Sandmaier BM, Storer B, et al. Myeloabltative vs myeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia 2006;20:128ŌĆō135PMID : 16270037.


32. Nagler A, Slavin S, Varadi G, et al. Allogeneic peripheral blood stem cell transplantation using a fludarabine-based low intensity conditioning regimen for malignant lymphoma. Bone Marrow Transplant 2000;25:1021ŌĆō1028PMID : 10828860.


33. Michallet M, Bilger K, Gargan F, et al. Allogeneic hematopoietic stem-cell transplantation after nonmyeloablative preparative regimens: impact of pretransplantation and posttransplantation factors on outcome. J Clin Oncol 2001;19:3340ŌĆō3349PMID : 11454881.


34. Baron F, Storb R, Storer BE, et al. Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopoietic cell transplantation. J Clin Oncol 2006;24:4150ŌĆō4157PMID : 16896000.


35. Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol 2005;23:1993ŌĆō2003PMID : 15774790.


36. Maruyama D, Fukuda T, Kato R, et al. Comparable antileukemia/lymphoma effects in nonremission patients undergoing allogeneic hematopoietic cell transplantation with a conventional cytoreductive or reduced-intensity regimen. Biol Blood Marrow Transplant 2007;13:932ŌĆō941PMID : 17640597.


37. Hegenbart U, Niederwieser D, Sandmaier BM, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol 2006;24:444ŌĆō453PMID : 16344316.


38. Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant 2006;12:1047ŌĆō1055PMID : 17067911.


39. Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood 2007;109:1395ŌĆō1400PMID : 17038533.


40. Or R, Shapira MY, Resnick I, et al. Nonmyeloablative allogeneic stem cell transplantation for the treatment of chronic myeloid leukemia in first chronic phase. Blood 2003;101:441ŌĆō445PMID : 12393604.


41. Crawley C, Szydlo R, Lalancette M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood 2005;106:2969ŌĆō2976PMID : 15998838.


42. Baron F, Maris MB, Storer BE, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with chronic myeloid leukemia. Biol Blood Marrow Transplant 2005;11:272ŌĆō279PMID : 15812392.


43. Martino R, Giralt S, Caballero MD, et al. Allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning in acute lymphoblastic leukemia: a feasibility study. Haematologica 2003;88:555ŌĆō560PMID : 12745275.

44. Hamaki T, Kami M, Kanda Y, et al. Reduced-intensity stem-cell transplantation for adult acute lymphoblastic leukemia: a retrospective study of 33 patients. Bone Marrow Transplant 2005;35:549ŌĆō556PMID : 15756282.


45. Lee S, Cho BS, Kim SY, et al. Allogeneic stem cell transplantation in first complete remission enhances graft-versus-leukemia effect in adults with acute lymphoblastic leukemia: antileukemic activity of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2007;13:1083ŌĆō1094PMID : 17697971.


46. Imamura M, Asano S, Harada M, et al. Current status of hematopoietic cell transplantation for adult patients with hematologic diseases and solid tumors in Japan. Int J Hematol 2006;83:164ŌĆō178PMID : 16513537.


47. Shigematsu A, Kondo T, Yamamoto S, et al. Exellent outcome of allogeneic hematopoietic stem cell transplantation using a conditioning regimen with medium-dose VP-16, cyclophosphamide and total body irradiation for adult patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2008;14:568ŌĆō575PMID : 18410899.


48. Gribben JG, Zahrich D, Stephans K, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood 2005;106:4389ŌĆō4396PMID : 16131571.



49. Rondon G, Giralt S, Huh Y, et al. Graft-versus-leukemia effect after allogeneic bone marrow transplantation for chronic lymphocytic leukemia. Bone Marrow Transplant 1996;18:669ŌĆō672PMID : 8879640.

50. Dreger P, Brand R, Milligan D, et al. Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: a population-matched analysis. Leukemia 2005;19:1029ŌĆō1033PMID : 15830011.


51. Corradini P, Tarella C, Olivieri A, et al. Reduced-intenisty conditioning followed by allografting of hematopoietic cells can produce clinical and molecular remissions in patients with poor-risk hematologic malignancies. Blood 2002;99:75ŌĆō82PMID : 11756155.


52. Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Blood 2002;100:4310ŌĆō4316PMID : 12393626.


53. Khouri IF, Saliba RM, Giralt SA, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host diseases, and transplant-related mortality. Blood 2001;98:3595ŌĆō3599PMID : 11739162.


54. Bjorkstrand BB, Ljungman P, Svensson H, et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood 1996;88:4711ŌĆō4718PMID : 8977265.


55. Tricot G, Vesole DH, Jagannath S, et al. Graft-versus-myeloma effect: proof of principle. Blood 1996;87:1196ŌĆō1198PMID : 8562947.


56. Martinelli G, Terragna C, Zamagni E, et al. Molecular remission after allogeneic or autologous transplantation of hematopoietic stem cells for multiple myeloma. J Clin Oncol 2000;18:2273ŌĆō2281PMID : 10829048.


57. Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem cell transplantation for multiple myeloma. N Engl J Med 2003;349:2495ŌĆō2502PMID : 14695409.


58. Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol 2007;25:2434ŌĆō2441PMID : 17485707.


59. Kruger N, Schwerdtfeger R, Kiehl M, et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood 2002;100:755ŌĆō760PMID : 12130482.


60. Maloney DG, Molina AJ, Sahebi F, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood 2003;102:3447ŌĆō3454PMID : 12855572.


61. Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood 2006;107:3474ŌĆō3480PMID : 16397129.


62. Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 2006;24:431ŌĆō436PMID : 16365178.


63. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005;452:2487ŌĆō2498PMID : 15958804.



64. Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with tahildomide improves survival in patients with multiple myeloma. Blood 2006;108:3289ŌĆō3294PMID : 16873668.


65. Kolb HJ, Shattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995;86:2041ŌĆō2050PMID : 7655033.


66. Porter DL, Connors JM, van Deerlin VM, et al. Graft-versus-tumor induction with donor leukocyte infusions as primary therapy for patients with malignancies. J Clin Oncol 1999;17:1234. PMID : 10561184.


67. Dazzi F, Szydlo RM, Cross NC, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood 2000;96:2712ŌĆō2716PMID : 11023502.


68. Lokhorst HM, Schattenberg A, Cornelissen JJ, et al. Donor leukocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation. Blood 1997;90:4206ŌĆō4211PMID : 9354693.


69. Salama M, Nevill T, Marcellus D, et al. Donor leukocyte infusions for multiple myeloma. Bone Marrow Transplant 2000;26:1179ŌĆō1184PMID : 11149728.


70. Lokhorst HM, Schattenberg A, Cornelissen JJ, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol 2000;18:3031ŌĆō3037PMID : 10944138.


71. Bardos A, Barlogie B, Morris C, et al. High response rate in refractory and poor-risk multiple myeloma after allotransplantation using a nonmyeloablative conditioning regimen and donor lymphocyte infusions. Blood 2001;97:2574ŌĆō2579PMID : 11313244.


72. Alyea E, Weller E, Schlossman R, et al. T-cell-depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood 2001;98:934ŌĆō939PMID : 11493435.


73. Ratanatharthorn V, Uberti J, Karanes C, et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin's lymphoma. Blood 1994;84:1050ŌĆō1055PMID : 8049425.


74. Jones RJ, Ambinder RF, Piantadosi S, Santos GW. Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood 1991;77:649ŌĆō653PMID : 1991174.


75. Mandigers CM, Meijerink JP, Raemaekers JM, et al. Graft versus-lymphoma effect of donor leukocyte infusion shown by real-time quantitative PCR analysis of t(14;18). Lancet 1998;352:1522ŌĆō1523PMID : 9820306.


76. Porter DL, Stadtmauer EA, Lazarus HM. 'GVHD': graft-versu-shost disease or graft-versus-Hodgkin's disease? An old acronym with new meaning. Bone Marrow Transplant 2003;31:739ŌĆō746PMID : 12732878.


77. Marks DI, Lush R, Cavenagh J, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood 2002;100:3108ŌĆō3114PMID : 12384406.


78. Bethge WA, Hegenbart U, Stuart MJ, et al. Adoptive immunotherapy with donor lymphocyte infusions after allogeneic hematopietic cell transplantation following nonmyeloablative conditioning. Blood 2004;103:790ŌĆō795PMID : 14525766.


79. Miura Y, Tanaka J, Toubai T, et al. Analysis of donor type chimerism in lineage-specific cell populations after allogeneic myeloablative and nonmyeloablative cell transplantation. Bone Marrow Transplant 2006;37:837ŌĆō843PMID : 16547484.


80. Baron F, Baker JE, Strob R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 2004;104:2254ŌĆō2262PMID : 15226174.


81. Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts: II. Rejection of parental grafts by resistant F1 hybrid mice. J Exp Med 1971;134:1513ŌĆō1528PMID : 4942407.



82. Murphy WJ, Kumar V, Bennett M. Acute rejection of murine bone marrow allografts by natural killer cells and T cells: differences in kinetics and target antigens recognized. J Exp Med 1987;166:1499ŌĆō1509PMID : 3316472.



83. Cho SG, Shuto Y, Soda Y, et al. Anti-NK cell treatment induces stable mixed chimerism in MHC-mismatched, T cell-depleted, nonmyeloablative bone marrow transplantation. Exp Hematol 2004;32:1246ŌĆō1254PMID : 15588949.


84. Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic stem cell transplantation with nonmyeloablative conditioning. J Clin Oncol 2005;23:1993ŌĆō2003PMID : 15774790.


85. Chan GW, Gorgun G, Miller KB, Foss FM. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Biol Blood Marrow Transplant 2003;9:170ŌĆō176PMID : 12652467.


86. Chen W, Masterman KA, Basta S, et al. Cross-priming of CD8+ T cells by viral and tumor antigens is a robust phenomenon. Eur J Immunol 2004;34:194ŌĆō199PMID : 14971045.


87. Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med 2004;10:987ŌĆō992PMID : 15286785.


88. Reddy P, Maeda Y, Liu C, et al. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med 2005;11:1244ŌĆō1249PMID : 16227991.


89. Anderson BE, McNiff JM, Jian D, et al. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood 2005;105:2227ŌĆō2234PMID : 15522961.


90. Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 1999;285:412ŌĆō415PMID : 10411505.


91. Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295:2097ŌĆō2100PMID : 11896281.


92. Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4:B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood 1994;83:3815ŌĆō3825PMID : 7515723.


93. Blazar BR, Taylor PA, Panoskaltsis-Mortari A, et al. Coblockade of the LFA1:ICAM1 and CD28/CTLA4:B7 pathways is a highly effective means of preventing graft-versushost disease induced by fully major histocompatibility complex-disparate donor grafts. Blood 1995;85:2607ŌĆō2618PMID : 7537122.


94. Blazar BR, Taylor PA, Boyer MW, et al. CD28/B7 interactions are required for sustaining the graft-versus-leukemia effect of delayed post-bone marrow transplantation splenocyte infusion in murine recipients of myeloid or lymphoid leukemia cells. J Immunol 1997;159:3460ŌĆō3473PMID : 9317145.



95. Ohata J, Sakurai J, Saito K, et al. Differential graft-versus-leukaemia effect by CD28 and CD40 costimulatory blockade after graft-versus-host disease prophylaxis. Clin Exp Immunol 2002;129:61ŌĆō68PMID : 12100023.



96. Blazar BR, Sharpe AH, Chen AI, et al. Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients. Blood 2003;101:3741ŌĆō3748PMID : 12521997.


97. Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells downregulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM). Blood 2005;105:3372ŌĆō3380PMID : 15618467.


98. Liang Y, Liu C, Djeu JY, et al. Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effect. Blood 2008;111:954ŌĆō962PMID : 17928532.



99. Murai M, Yoneyama H, Harada A, et al. Active participation of CCR5+CD8+ T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest 1999;104:49ŌĆō57PMID : 10393698.



100. Serody JS, Burkett SE, Panoskaltsis-Mortari A, et al. T-lymphocyte production of macrophage inflammatory protein-1 alpha is critical to the recruitment of CD8+ T cells to the liver, lung, and spleen during graft-versus-host disease. Blood 2000;96:2973ŌĆō2980PMID : 11049973.


101. New JY, Li B, Koh WP, et al. T cell infiltration and chemokine expression: relevance to the disease localization in murine graft-versus-host disease. Bone Marrow Transplant 2002;29:979ŌĆō986PMID : 12098066.


102. Sasaki M, Hasegawa H, Kohno M, et al. Antagonist of secondary lymphoid-tissue chemokine (CCR ligand 21) prevents the development of chronic graft-versus-host disease in mice. J Immunol 2003;170:588ŌĆō596PMID : 12496447.


103. Duffner U, Lu B, Hildebrandt GC, et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol 2003;31:897ŌĆō902PMID : 14550805.


104. Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood 2005;106:3300ŌĆō3307PMID : 16002422.



105. Wysocki CA, Panoslkaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood 2005;105:4191ŌĆō4199PMID : 15701715.



106. Rao AR, Quinones MP, Garavito E, et al. CC chemokine receptor 2 expression in donor cells serves an essential role in graft-versus-host disease. J Immunol 2003;171:4875ŌĆō4885PMID : 14568968.


107. Terwey TH, Kim TD, Kochman AA, et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood 2005;106:3322ŌĆō3330PMID : 16037386.



108. Choi SW, Hildebrandt GC, Olkiewicz KM, et al. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood 2007;110:3447ŌĆō3455PMID : 17641205.



109. Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A 2004;101:8120ŌĆō8125PMID : 15148407.



110. Reddy P, Maeda Y, Hotary K, et al. Histon deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemic effect. Proc Natl Acad Sci U S A 2004;101:3921ŌĆō3926PMID : 15001702.



111. Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 1999;94:333ŌĆō339PMID : 10381530.


112. Igarashi T, Wynberg J, Srinivasan R, et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood 2004;104:170ŌĆō177PMID : 15016654.


113. Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105:3051ŌĆō3057PMID : 15632206.


114. Antin JH, Kim HT, Cutler C, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood 2003;102:1601ŌĆō1605PMID : 12730113.


115. Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2004;10:328ŌĆō336PMID : 15111932.


116. Hori T, Naishiro Y, Sohma H, et al. CCL8 is a potential molecular candidate for the diagnosis of graft-versus-host disease. Blood 2008;111:4403ŌĆō4412PMID : 18256320.



117. Ota Y, Yamamoto M, Hori T, et al. Upregulation of plasma CCL8 in mouse model of graft-versus-host disease. Exp Hematol 2009;37:525ŌĆō531PMID : 19302923.


118. Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood 2009;113:273ŌĆō278PMID : 18832652.



119. Goulmy E, Schipper R, Pool J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med 1996;334:281ŌĆō285PMID : 8532022.


120. Marijit WA, Heemskek MH, Kloosterboer FM, et al. Hmatopoiesis restricted minor histocompatibility antigens HA-1- or HA-2specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci U S A 2003;100:2742ŌĆō2747PMID : 12601144.



121. Spierings E, Brigitte W, Goulmy E. Minor histocompatibility antigens: big in tumor therapy. Trends Immunol 2004;25:56ŌĆō60PMID : 15102363.


122. Warren EH, Gavin MA, Simpson E, et al. The human UTY gene encodes a novel HLA-B8-restricted H-Y antigen. J Immunol 2000;164:2807ŌĆō2814PMID : 10679124.


123. Dickinson AM, Wang XN, Sviland L, et al. In situ dissection of the graft-versus-host activities of cytotoxic T cells specific for minor histocompatibility antigens. Nat Med 2002;8:410ŌĆō414PMID : 11927949.


124. Zorn E, Miklos DB, Floyd BH, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med 2004;199:1133ŌĆō1142PMID : 15096539.



125. Rosinski KV, Fujii N, Mito JK, et al. DDX3Y encodes a class I MHC-restricted H-Y antigen that is expressed in leukemic stem cells. Blood 2008;111:4817ŌĆō4826PMID : 18299450.



126. Molldrem JJ, Lee PP, Wang C, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med 2000;6:1018ŌĆō1023PMID : 10973322.


127. Molldrem JJ, Komanduri K, Wieder E. Overexpressed diffenerntiation antigens as targets of graft-versus-leukemia reactions. Curr Opin Hematol 2002;9:503ŌĆō508PMID : 12394172.


128. Yong AS, Rezvani K, Savani BN, et al. High PR3 or ELA2 expression by CD34+ cells in advanced-phase chronic myeloid leukemia is associated with improved outcome following allogeneic stem cells transplantation and may improve PR1 peptide-driven graft-versus-leukemia effects. Blood 2007;110:770ŌĆō775PMID : 17412886.



129. Gao L, Bellantuono I, Elsassser A, et al. Selective elimination of leukemic CD34+ progenitor cell by cytotoxic T lymphocytes specific for WT1. Blood 2000;95:2198ŌĆō2203PMID : 10733485.


130. Gaiger A, Reese V, Disis ML, Cheever MA. Immunity to WT1 in the animal models and in patients with acute myeloid leukemia. Blood 2000;96:1480ŌĆō1489PMID : 10942395.


131. Oka Y, Elisseeva OA, Tsuboi A, et al. Human cytotoxic Tlymphocyte responses specific for peptides of the wild-type Wilms' tumor gene (WT1) product. Immunogenetics 2000;51:99ŌĆō107PMID : 10663572.


132. Azuma T, Otsuki T, Kuzushima K, et al. Myeloma cells are highly sensitive to the granule exocytosis pathway mediated by WT1-specific cytotoxic T lymphocytes. Clin Cancer Res 2004;10:7402ŌĆō7412PMID : 15534117.


133. Fukuda S, Singh P, Moh A, et al. Survivin mediates aberrant hematopoietic progenitor cell proliferation and acute leukemia in mice induced by internal tandem duplication of Flt3. Blood 2009;114:394ŌĆō403PMID : 19411632.



134. Reker S, Meier A, Holten-Andersen L, et al. Identification of novel surviving-derived CTL epitope. Cancer Biol Ther 2004;3:173ŌĆō179PMID : 14726703.


135. Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, surviving, expressed in cancer and lymphoma. Nat Med 1997;3:917ŌĆō921PMID : 9256286.


136. Schwartz J, Pinilla-Ibarz RR, Scheinberg DA. Novel targeted and immunotherapeutic strategies in chronic myeloid leukemia. Semin Hematol 2003;40:87ŌĆō96PMID : 12563615.


137. Kwak LW, Taub DD, Duffey PL, et al. Transfer of myeloma idiotype-specific immunity from an actively immunized marrow donor. Lancet 1995;345:1016ŌĆō1020PMID : 7723498.


138. Yotnda P, Firat H, Garcia-Pons F, et al. Cytotoxic T cell response against the chimeric p210 BCR-ABL protein in patients with chronic myelogenous leukemia. J Clin Invest 1998;101:2290ŌĆō2296PMID : 9593785.



139. Yong AS, Keyvanfar K, Eniafe R, et al. Hematopoietic stem cells and progenitors of chronic myeloid leukemia express leukemia associated antigens: implications for the graft-versus-leukemia effect and peptide-based immunotherapy. Leukemia 2008;22:1721ŌĆō1727PMID : 18548092.



Table┬Ā2
Anti-hematological malignancy effects by NST and DLI
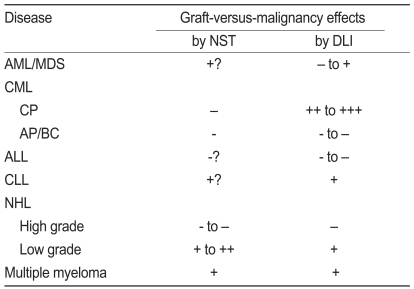
NST, nonmyeloablative stem cell transplantation; DLI, donor lymphocyte infusion; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; CP, chronic phase; AP, accelerated phase; BC, blast crisis; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin's lymphoma.
-
METRICS

- Related articles
-
Sexuality and Quality of Life after Hematopoietic Stem Cell Transplantation2002 March;17(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


