 |
 |
| Korean J Intern Med > Volume 24(3); 2009 > Article |
|
Abstract
Background/Aims
The efficacy and safety of a combination of ezetimibe and low-dose statin as primary treatment for dyslipidemia in renal transplant patients were evaluated prospectively.
Methods
The study enrolled 77 renal transplant recipients with dyslipidemia. They were given ezetimibe (10 mg) and simvastatin (10 mg) for 6 months as the initial treatment for dyslipidemia. Efficacy and safety were evaluated using lipid profiles, trough calcineurin inhibitor levels, allograft function, and adverse effects. The effects on proteinuria and high sensitivity C-reactive protein (hsCRP) levels were also evaluated.
Results
Ezetimibe and low-dose simvastatin significantly decreased the levels of total cholesterol (34.6%), triglyceride (16.0%), and low-density lipoprotein cholesterol (LDL-C) (47.6%), and 82.5% of the patients reached the target LDL-C level of <100 mg/dL. No significant change in the trough calcineurin inhibitor levels or allograft function occurred, and no serious adverse effects were observed. Fourteen patients (18.2%) discontinued treatment; eight patients (11.7%) developed muscle pain or weakness without an increase in creatinine kinase levels, and two patients (2.6%) developed elevated liver transaminase levels. The proteinuria and hsCRP levels did not change significantly.
Dyslipidemia, a major complication in renal transplant patients, increases the risk of cardiovascular disease (CVD) [1,2], which is a major cause of morbidity and mortality in the renal transplant population [3]. The clinical practice guidelines of the National Kidney Foundation Work Group recommend a target low-density lipoprotein cholesterol (LDL-C) level of less than 100 mg/dL [4], and the use of a statin as the first-line treatment. Nevertheless, in clinical practice, many patients fail to reach the target LDL-C level. The reasons for this may be multifactorial, but include a tendency to reduce the statin dose because of concerns about the adverse effects related to higher doses and drug interactions between the statin and calcineurin inhibitors (CNIs) [4,5].
Ezetimibe lowers cholesterol levels by inhibiting the intestinal absorption of cholesterol [6] so ezetimibe is especially useful for patients who are statin-intolerant or resistant to high-dose statin monotherapy [7]. The incremental lipid-lowering effect of ezetimibe has been observed in both non-transplant [8-18] and renal transplant populations [19-22]. However, no report has examined the use of ezetimibe combined with a low-dose statin as the initial treatment in renal transplant patients.
This study prospectively evaluated the efficacy and safety of ezetimibe and low-dose simvastatin therapy as primary treatment for dyslipidemia in renal transplant recipients. Our results revealed that primary treatment of renal transplant recipients with ezetimibe and low-dose simvastatin lowers lipid levels dramatically without significant adverse reactions.
A prospective study examined renal transplant recipients receiving a single combination tablet of ezetimibe (10 mg) and simvastatin (10 mg) daily. The inclusion criteria were the passage of at least 4 months after renal transplantation, fasting LDL-C levels over 100 mg/dL despite therapeutic lifestyle changes, and informed consent of the patient. Patients who had one or more of the following criteria were excluded: use of other lipid-lowering drugs (including statin monotherapy) within 4 weeks of the start of ezetimibe and simvastatin therapy, an episode of unstable angina or myocardial infarction within the past 6 months, preexisting malignancy with a life expectancy of less than 1 year, abnormal liver enzymes (aspartate aminotransferase [AST] or alanine aminotransferase [ALT]) ≥2× the upper limit of normal, and pregnant or breast-feeding women.
After a 1-month screening period, eligible patients were enrolled in the study. The doses of simvastatin and ezetimibe were not changed during the study period. Study visits were scheduled before initiating treatment and after 2 weeks and 1, 3, and 6 months. Fasting lipid profiles (total cholesterol [TC], triglyceride [TG], LDL-C, and high-density lipoprotein cholesterol [HDL-C]), creatinine kinase (CK), and trough immunosuppressant levels (cyclosporin A [CsA], tacrolimus [Tac], and sirolimus [SRL]) were measured at all scheduled visits. The urine protein-to-creatinine ratio in a spot urine sample, liver transaminase (AST, ALT), and high sensitivity C-reactive protein (hsCRP) were measured before initiating treatment and after 1, 3, and 6 months. Adverse drug reactions, such as myalgia and skin rash, were monitored at all scheduled visits. Treatment compliance was assessed after 1, 3, and 6 months. The study protocol was approved by the institutional review board.
The following variables were analyzed before and after ezetimibe and low-dose simvastatin treatment: fasting lipid profiles (TC, TG, LDL-C, and HDL-C), estimated glomerular filtration rate (eGFR), urine protein-to-creatinine ratio in a spot urine sample, liver transaminase (AST, ALT), hsCRP, CK, trough immunosuppressant levels (CsA, Tac, and SRL), and adverse drug reactions. LDL-C was calculated using Friedewald's equation (LDL-C=TC-[TG/5]-[HDL-C] and eGFR was calculated using the abbreviated Modification of Diet and Renal Disease (MDRD) equation [23].
Continuous data are presented as the median and range or mean±SD and were compared using the paired t-test. In a subgroup analysis of patients with proteinuria (≥500 mg/g) or high hsCRP levels (≥0.5 mg/dL), data including proteinuria and hsCRP levels were compared using the Wilcoxon signed-rank test.
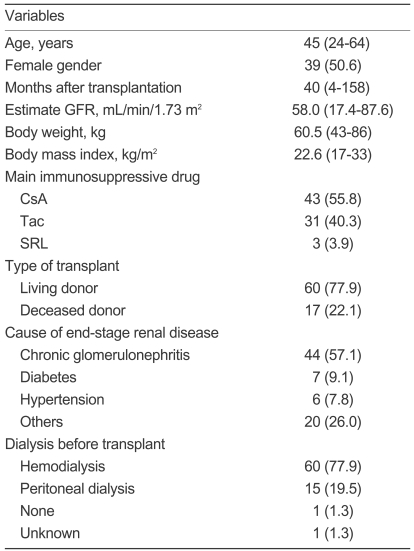
Seventy-seven patients were enrolled in this study. The patient characteristics are summarized in Table 1. Three patients were on CNI-free regimens (SRL and prednisolone) and two patients were on corticosteroid-free regimens (CsA and mycophenolate mofetil). Fifty-six patients were on a triple immunosuppressive regimen and 21 patients were on a dual immunosuppressive regimen. Sixty-three patients (81.8%) continued on ezetimibe and low-dose simvastatin therapy for the full 6 months.
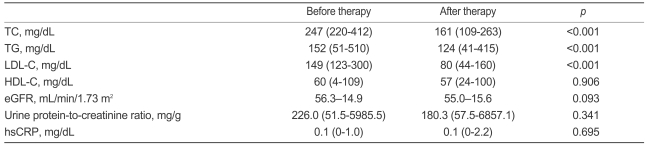
The overall effects of treatment on the fasting lipid profiles are shown in Table 2. The TC, TG, and LDL-C levels were reduced significantly after treatment with mean percentage changes of 34.6±9.5, 16.0±27.4, and 47.6±13.7%, respectively. After 6 months of treatment, 52 patients (82.5%) reached the target of LDL-C <100 mg/dL.
An analysis of subgroups divided according to the type of CNI showed that the lipid-lowering effect was significant in both the CsA and Tac groups (Table 3). In CsA-treated patients, the levels of TC, TG, and LDL-C were reduced significantly with mean percentage changes of 33.9±6.5, 12.0±29.5, and 47.2±9.9%, respectively. In Tac-treated patients, the TC, TG, and LDL-C levels were significantly reduced with mean percentage changes of 35.8±12.0, 21.4±24.5, and 48.8±16.7%, respectively. Stable trough CsA and Tac levels were maintained. One patient was on SRL and had a trough level of 8.9 ng/mL before treatment and 9.5 ng/mL after treatment.
As shown in Table 2, no significant change occurred in the eGFR, urine protein-to-creatinine ratio, or hsCRP concentration after treatment. In a subgroup analysis of patients with proteinuria of at least 500 mg/g (n=16), the median urine protein-to-creatinine ratio was reduced from 1558.5 (range 500.4-5985.5) to 869.5 (range 93.2-6857.1) mg/g, but the difference was not significant (p=0.14). In a subgroup analysis of patients with hsCRP levels ≥0.5 mg/dL (n=6), the median hsCRP concentration was reduced from 0.7 (range 0.5-1.0) to 0.3 (range 0.02-2.2) mg/dL, but statistical significance was not achieved (p= 0.6).
Fourteen patients (18.2%) discontinued the ezetimibe and low-dose simvastatin treatment. The most common reason was muscle pain or weakness (n=8, 11.7%) without a significant increase in CK levels. Two patients (2.6%) had greater than twofold increases in the upper limit levels of AST and ALT. Other reasons were skin rash, dizziness, pregnancy, and concomitant administration of itraconazole.
Our results clearly demonstrate the lipid-lowering efficacy of a combination of ezetimibe and low-dose statin in renal transplant recipients with dyslipidemia. Four reports on the lipid-lowering efficacy of ezetimibe in renal transplant patients have been published [19-22], but these studies focused mainly on patients who were statin-intolerant or resistant to high-dose statin monotherapy. Compared to previous reports, our study provides evidence of the potential advantage of the combination of ezetimibe and low-dose statin as the initial treatment for dyslipidemia in this population.
The most important finding of this study is that treatment with a combination of ezetimibe and low-dose statin effectively lowered the LDL-C level in renal transplant recipients. With ezetimibe (10 mg) and simvastatin (10 mg), the LDL-C level was reduced 47.6% on average and 82.5% of the patients reached the LDL-C goal of <100 mg/dL (Table 2). Our results (47.6% reduction in the LDL-C level) are better than those of a study on statin monotherapy (fluvastatin, 40 mg) in a large number of renal transplant patients (32% reduction in the LDL-C level) [24]. Furthermore, we obtained better results than studies reporting an average 31% reduction (range 22-41%) in the LDL-C level using ezetimibe with or without high-dose statin treatment [19-22]. The reason for the enhanced lipid-lowering effect seen in our study may be explained partially by the higher pretreatment LDL-C levels [20,22]. Nevertheless, it suggests that initial treatment with ezetimibe and low-dose statin has a more potent lipid-lowering effect than other regimens, such as statin monotherapy or the secondary addition of ezetimibe to a statin. This presumption is supported by the excellent results (45-54%) in non-transplant patients initially treated with ezetimibe and a statin [11-13,16,18].
In addition to its excellent effect on dyslipidemia, renal transplant patients receiving a CNI would benefit from ezetimibe and low-dose statin therapy. The effective dose of ezetimibe is higher in patients receiving CsA than in patients not receiving CsA [25-27]. The mechanism of the interaction between ezetimibe and CsA is not fully understood, but CsA is thought to alter the glucuronidation of ezetimibe in gastrointestinal tissue, which alters the pharmacokinetics of ezetimibe and its metabolites [27]. Therefore, patients on CsA may have a higher lipid-lowering effect with lower doses of ezetimibe than those not receiving CsA.
Another advantage of the combination treatment with ezetimibe and low-dose statin is safety. In this study, the incidence of adverse reactions associated with statins was quite low. Only 2.6% of the patients developed elevated transaminase levels, which is a rate similar to that in the non-transplant population [28]. Muscle pain or weakness occurred in 11.7% of the patients, but it was nonspecific and was not associated with increased CK levels. The blood trough CNI levels and allograft function measured using the estimated GFR were stable during the 6-month treatment. This suggests that combination treatment with ezetimibe and low-dose statin has fewer adverse effects and induces less allograft dysfunction than high-dose statin therapy.
Our study has some limitations. First, it lacks randomization to compare ezetimibe plus low-dose statin with high-dose statin monotherapy. Second, the observation period was short and the study population was relatively small, which meant that we did not observe a beneficial effect of statin on reducing proteinuria or hsCRP levels.
In conclusion, the combination of ezetimibe and low-dose statin is an effective primary lipid-lowering therapy in renal transplant patients. This treatment may help more transplant patients to achieve the optimal LDL-C target compared to statin monotherapy.
References
1. Massy ZA, Kasiske BL. Post-transplant hyperlipidemia: mechanisms and management. J Am Soc Nephrol 1996;7:971–977PMID : 8829110.


2. Jardine AG, Fellström B, Logan JO, et al. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis 2005;46:529–536PMID : 16129216.


3. Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. J Am Soc Nephrol 1996;7:158–165PMID : 8808124.


4. Kasiske B, Cosio FG, Beto J, et al. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: a report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am J Transplant 2004;4(Suppl 7):13–53PMID : 15027968.

5. Akhlaghi F, McLachlan AJ, Keogh AM, Brown KF. Effect of simvastatin on cyclosporine unbound fraction and apparent blood clearance in heart transplant recipients. Br J Clin Pharmacol 1997;44:537–542PMID : 9431828.



6. Sudhop T, Lutjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 2002;106:1943–1948PMID : 12370217.


7. Stein EA. Managing dyslipidemia in the high-risk patient. Am J Cardiol 2002;89(5A):50C–57CPMID : 11779522.


8. Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol 2002;40:2125–2134PMID : 12505224.


9. Melani L, Mills R, Hassman D, et al. Efficacy and safety of ezetimibe coadministered with pravastatin in patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Eur Heart J 2003;24:717–728PMID : 12713766.


10. Ballantyne CM, Houri J, Notarbartolo A, LeBeaut AP, Sager P, Veltri EP. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003;107:2409–2415PMID : 12719279.


11. Ose L, Johnson-Levonas A, Reyes R, et al. A multi-centre, randomised, double-blind 14-week extension study examining the long-term safety and efficacy profile of the ezetimibe/simvastatin combination tablet. Int J Clin Pract 2007;61:1469–1480PMID : 17655686.


12. Pearson T, Ballantyne C, Sisk C, Shah A, Veltri E, Maccubbin D. Comparison of effects of ezetimibe/simvastatin versus simvastatin versus atorvastatin in reducing C-reactive protein and low-density lipoprotein cholesterol levels. Am J Cardiol 2007;99:1706–1713PMID : 17560879.


13. Landray M, Baigent C, Leaper C, et al. The second United Kingdom Heart and Renal Protection (UK-HARP-II) Study: a randomized controlled study of the biochemical safety and efficacy of adding ezetimibe to simvastatin as initial therapy among patients with CKD. Am J Kidney Dis 2006;47:385–395PMID : 16490616.


14. Roeters van Lennep HW, Liem AH, Dunselman PH, Dallinga-Thie GM, Zwinderman AH, Jukema JW. The efficacy of statin monotherapy uptitration versus switching to ezetimibe/simvastatin: results of the EASEGO study. Curr Med Res Opin 2008;24:685–694PMID : 18226326.


15. Gagne C, Bays H, Weiss S, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol 2002;90:1084–1091PMID : 12423708.


16. Sager PT, Melani L, Lipka L, et al. Effect of coadministration of ezetimibe and simvastatin on high-sensitivity C-reactive protein. Am J Cardiol 2003;92:1414–1418PMID : 14675576.


17. Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008;358:1431–1443PMID : 18376000.



18. Bae JW, Kim HS, Lee SC, Han KH, Jeon ES. The safety and efficacy of ezetimibe and simvastatin combination therapy in Korean patients with primary hypercholesterolemia. Korean J Med 2005;68:487–497.
19. Puthenparumpil JJ, Keough-Ryan T, Kiberd M, Lawen J, Kiberd BA. Treatment of hypercholesterolemia with ezetimibe in the kidney transplant population. Transplant Proc 2005;37:1033–1035PMID : 15848614.


20. Langone AJ, Chuang P. Ezetimibe in renal transplant patients with hyperlipidemia resistant to HMG-CoA reductase inhibitors. Transplantation 2006;81:804–807PMID : 16534487.


21. Kohnle M, Pietruck F, Kribben A, Philipp T, Heemann U, Witzke O. Ezetimibe for the treatment of uncontrolled hypercholesterolemia in patients with high-dose statin therapy after renal transplantation. Am J Transplant 2006;6:205–208PMID : 16433776.


22. Buchanan C, Smith L, Corbett J, Nelson E, Shihab F. A retrospective analysis of ezetimibe treatment in renal transplant recipients. Am J Transplant 2006;6:770–774PMID : 16539634.


23. Pöge U, Gerhardt T, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant 2005;5:1306–1311PMID : 15888034.


24. Holdaas H, Fellström B, Jardine AG, et al. Effect of fluvastation on cardiac outcomes in renal transplant recipients: a multi center, randomized, placebo-controlled trial. Lancet 2003;361:2024–2031PMID : 12814712.


25. Koshman SL, Lalonde LD, Burton I, Tymchak WJ, Pearson GJ. Supratherapeutic response to ezetimibe administered with cyclosporine. Ann Pharmacother 2005;39:1561–1565PMID : 16030077.


26. Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet 2005;44:467–494PMID : 15871634.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



