INTRODUCTION
Atrial septal defect in the middle-aged and elderly, which is associated with dilated main pulmonary artery, usually induces progressive pulmonary hypertension in cases of un-correction or delayed corrective surgery1ŌĆō3). Furthermore, the progressive pulmonary hypertension is associated with hemodynamic compromise of the left ventricle and sudden cardiac death due to the collapse of systemic circulation. However, the causes of sudden death in the patient with severe pulmonary hypertension have remained uncertain. Among the causes of death, the failure of coronary circulation might be one of the causes. In this case, we reviewed total occlusion of the left main coronary artery caused by extrinsic compression of the markedly dilated main pulmonary trunk4ŌĆō7) that was induced by progressive pulmonary hypertension due to delayed closure of the atrial septal defect.
CASE
A 34-year-old woman was admitted to the hospital because of recently aggravated dyspnea, general edema and abdominal distension for 5 months. In her past, she had no history of Kawasaki disease during childhood. However, nine years before admission, patch repair with PTFE was performed for the secondum type of atrial septal defect (ASD). Thereafter, she had been managed for pulmonary hypertension and aggravating right heart failure.
The preoperative values collected from her past medical records of the previous hospital included pulmonary/systolic flow ratio (Qp/Qs) of 2:1, pulmonary/systolic resistance ratio (Rp/Rs) of 0.35, pulmonary arterial systolic pressure (PASP) of 51 mmHg and the diameter of main pulmonary artery of 40 mm, which were suggestive of significant left to right shunt with moderate pulmonary hypertension, and normal coronary artery. As the PASP during the postoperative care was not decreased to the normal range, the doctor who operated on the patient cautioned and wrote on her record of the possibility of progressing pulmonary hypertension.
On admission, blood pressure and pulse rate were 110/70 mmHg and 85 beats per minute, respectively. On physical examination, there were engorged neck vein, soft abdominal distension, palpable liver, and pretibial pitting edema. Systolic murmur of grade III/VI was auscultated from her lower sternal border.
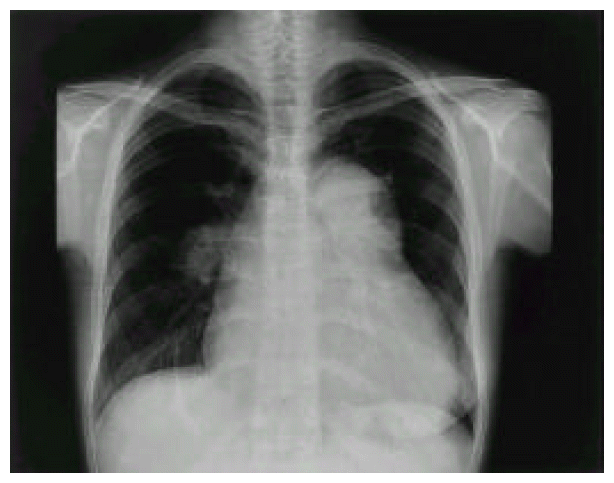
The arterial blood gas analysis showed pO2 of 77.4 mmHg, pCo2 of 31.2 mmHg under the room air. The rheumatoid factor (RF) and VDRL for syphilis were both negative. The electrocardiogram revealed normal sinus rhythm with incomplete right bundle branch block and biventricular hypertrophy. The chest PA showed markedly enlarged cardiac size with prominently dilated main pulmonary artery and centralization of pulmonary hilum, suggesting pulmonary arterial hypertension (Figure 1). Echocardiogram with Doppler study demonstrated right ventricular hypertrophy, D-shape left ventricle, as well as severe pulmonary and tricuspid regurgitation, which corresponded to right ventricular systolic pressure of 117 mmHg. However, there was no intracardiac shunt remained on color interrogation. Moreover, serial echocardiographic evaluation had revealed the progression of pulmonary hypertension after delivery of her baby.
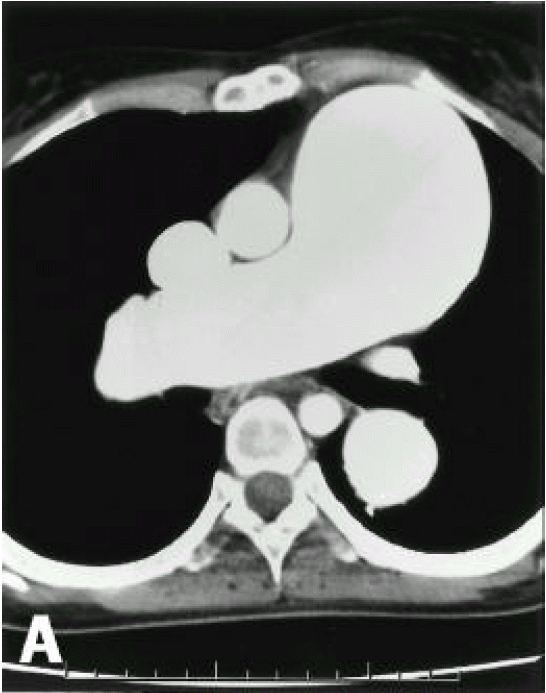
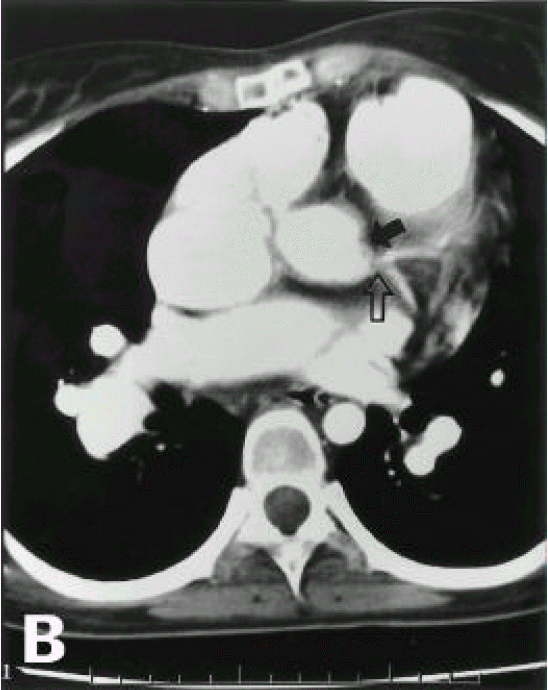
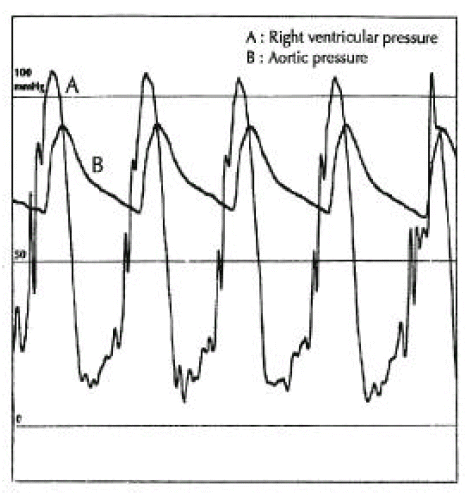
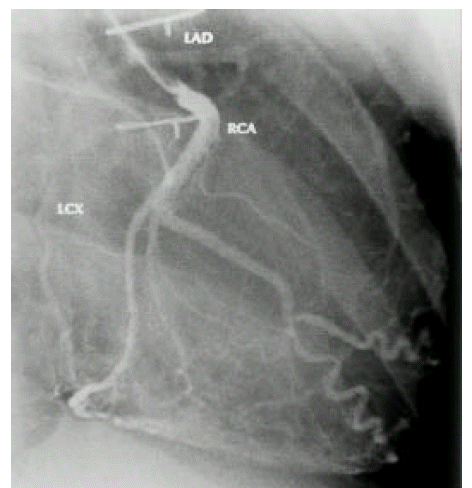
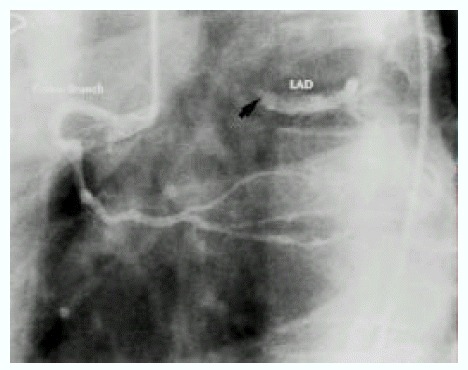
A diagnosis of Eisenmenger syndrome was made. The base-line study for heart-lung transplantation, including chest computed tomography (CT), cardiac catheterization and coronary angiography were undertaken. Chest CT scan with contrast revealed markedly dilated pulmonary trunk and both pulmonary arteries (Figure 2A), a concave disfigurement of the left side of the ascending aorta and nearly total occlusion the ostium of the left main coronary artery that was retrogradly filled with collateral circulation from the right coronary artery (Figure 2B). That was suggestive of compression of the left side of the aorta and the left main coronary artery together by the pulmonary trunk passing by the ascending aortaŌĆÖs left side. Cardiac catheterization revealed PASP of 105 mmHg, Qp/Qs of 1 and Rp/Rs of 1.4 (Figure 3), which mean no remaining shunt but much aggravated pulmonary hypertension compatible with Eisenmenger syndrome compared with preoperative data. The coronary angiography showed normal right coronary artery and the collaterals that came out from the conus branch to mid-left anterior descending artery (LAD) and that from the distal right coronary artery to the left circumflex artery (LCX) and to the distal LAD, respectively (Figure 4, 5). Engagement of the left main coronary artery with the diagnostic coronary catheter was not available. On aortography, the left main coronary artery was not visualized with no stump, suggesting total occlusion of the ostium of the left main coronary artery (Figure 6). There was no other luminal lesion in the remaining part of the coronary artery system and the thoracic as well as the abdominal aorta.
DISCUSSION
Some adults with congenital heart disease have associated ischemic heart disease3, 8). On the other hand, atherosclerosis9), syphilis10), aortitis11, 12) and congenital anomalies of the coronary artery13ŌĆō14) are widely-known causes of stenosis of the left main coronary trunk.
Compression of the left main coronary trunk has not been widely known. However, in 1957, Corday, et al.4) first pointed out that the distended main pulmonary artery easily compressed the left main coronary trunk because of the spatial position of the proximal part of the left coronary artery closed to the main pulmonary artery. Schaffer, et al.5) tried to prove the correlation between pulmonary pressure and coronary flow at autopsy which, however, was unsuccessful because the main pulmonary artery of the autopsied heart was not sufficiently dilated. Mitsudo et al.6) first reported the case of a female patient with ASD that was combined with severe narrowing of the ostium of the left main coronary trunk on angiography. After the patient died suddenly, the postmortem examination confirmed no anatomic or pathological abnormality of the coronary arteries. Also, they reported that, in adults with ASD and pulmonary hypertension, the coronary angiography revealed localized concave narrowing of the left main coronary trunk orifice in 7 (44%) of 16 patients. In patients without pulmonary hypertension, however, these findings were not observed.
Fijiwara et al.7) reported 3 cases of adult ASD with compression of the left main coronary trunk by dilated main pulmonary artery. In one patient of the three, magnetic resonance imaging study demonstrated that the left main coronary trunk ran close to the left main pulmonary artery so that the markedly distended pulmonary artery easily compressed the left main coronary trunk. In two of the three cases, one case died because of septicemia after closure of ASD, the narrowed left main coronary artery was no longer narrowed or improved the severity of narrowing after closure of ASD.
In our case, pulmonary hypertension due to ASD had already been present before the corrective surgery and it was progressively aggravated without improvement after the correction of the shunt up to the current admission. Therefore, the left main coronary artery had been continuously compressed, in spite of the post-closure status for ASD. We could rule out the other possible reasons for the negative results of the serologic tests for syphilis, other rheumatoid diseases and the absence of other manifestations of systemic vasculitis. The reason for disfigurement of the left side ascending aorta and the ostium of the left main coronary artery was considered to be an extrinsic compression owing to extremely dilated pulmonary artery (Figure 2B).
In stenosis of the left main coronary artery, as the mortality rate of patients without surgical treatment is high15), aorto-coronary artery bypass is absolutely indicated6). In our case, however, the left coronary artery, including the left main artery, was fully visualized with collaterals of grade III from the right coronary artery (Figure 4, 5) which explains that she seldom suffered from angina. It is possible to say that long-standing, slowly progressive narrowing of the left main coronary artery occlusion induces the development of sufficient collateral circulation without severe myocardial ischemia. Fijiwara et al7) suggested that narrowing of the left main coronary trunk improved or disappeared after ASD closure, thanks to normalization of pulmonary flow. That means if the pulmonary hypertension is reversible, aorto-coronary bypass may be unnecessary. However, in our experience, the reversibility of the narrowing of the left coronary artery was not only determined by the ASD closure but also by the time of the closure. The narrowing of the left coronary artery may not be relieved after operation, if it is performed too late like our case.
We concluded, in agreement with previous reported cases4ŌĆō7), that the dilated pulmonary artery trunk due to ASD associated with pulmonary hypertension can be a possible cause of left main coronary artery occlusion and that, if the ASD closure was too late, the narrowing or occlusion of the left coronary artery could not be resolved, even after operation, owing to irreversible pulmonary hypertension.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





