 |
 |
| Korean J Intern Med > Volume 16(3); 2001 > Article |
|
Abstract
Background :
Hypersecretion of mucin due to goblet cell hyperplasia is frequently encountered in many chronic airway diseases, such as chronic bronchitis, bronchiectasis, bronchial asthma and cystic fibrosis. Even in normal individuals, viral infection or bacterial pneumonia frequently provoke huge amounts of bronchial secretions which may cause airway obstruction. The production of mucin was regulated by epidermal growth factor (EGF) in vitro2). To know whether this EGF system regulates mucin secretion in vivo and Pseudomonas also stimulates the mucin secretion by the same pathway, we studied these relationships in the cultured rat tracheal epithelial cells.
Methods :
Rat tracheal epithelial cells were obtained by pronase dissociation from the male Fisher 344 rats. When cells became confluent, they were divided into 6 groups and stimulated with either EGF for 24 hours or Pseudomonas extracts for 12 hours with or without selective EGF-R tyrosine kinase inhibitor tyrphostin AG 1478.
Results :
We found that both EGF and Pseudomonas extracts phosphorylated the tyrosine residue in the EGF receptor from the rat tracheal epithelial cells and this tyrosine phosphorylation was nearly completely blocked by selective EGF-R tyrosine kinase inhibitor tyrphostin AG 1478. The mucin secretion was also stimulated by either EGF or Pseudomonas extracts but more strong secretion of mucin and MUC5AC gene expression in the rat tracheal epithelial cell was done by Pseudomonas extracts.
Mucus hypersecretion is a frequent finding in various inflammatory airway diseases, such as viral or bacterial airway infections, bronchial asthma, bronchiectasis, cystic fibrosis and chronic bronchitis. Mucin glycoproteins, the major macromolecular constituents of mucus, impart viscoelastic qualities to mucus. They are large, heavily O-glycosylated molecules and have been difficult to characterize biochemically. Nine mucin genes have been identified and are expressed as mRNA in human respiratory epithelium (MUC1-4, MUC5AC, MUC5B, and MUC6-8). Of these, MUC5AC is the only mucin gene product isolated from normal human airway secretions and is, therefore, proposed to be a major airway secretory mucin1). It has been reported that epidermal growth factor receptor (EGFR) activation by its ligands leads to mucin MUC5AC synthesis and goblet cell production in human bronchial epithelial cells in vitro2). EGFR tyrosine phosphorylation promotes its association with signaling proteins, leads to membrane-associated Ras activation and initiates downstream signaling to the nucleus3). Pseudomonas aeruginosa also increases the mucin secretion and upregulates the MUC2 mucin transcription in NCIH292 cells5).
The purpose of the studies reported here was to determine whether EGF or Pseudomonas increase the mucin secretion and MUC5AC gene expression by way of the EGFR in the rat tracheal epithelial cells.
Isolated rat trachea was incubated with 0.1% pronase overnight. Tracheal epithelial cells were harvested through the flushing of the trachea with 10% FBS containing s-MEM solution. After washing, cells were resuspended in 5% FBS containing M/D + 6F (insulin 5 ug/mL, transferrin 5 ug/mL, epidermal growth factor 12.5 ng/mL, hydrocortisone 10ŌłÆ7 M, selenite 10ŌłÆ8 M, retinoic acid 10ŌłÆ7 M, fungisone 250 ug/mL) solution. Cells were cultured in the collagen gel (Vitrogen-100) coated petri dishes until confluence. Cells were switched to no serum medium for 24 hours and then stimulated with either EGF (50 ng/mL, for 24 hours) or Pseudomonas extract (1:40, for 12 hours). In inhibition studies, cells were pretreated with selective EGF-R tyrosine kinase inhibitors, tyrphostin AG 1478 (10 uM) 30 minutes before adding stimulants.
Pseudomonas aeruginosa strain of PAO1 was grown in M9 buffer for 72 h at 37 ┬░C. Cell-free supernatant was obtained by centrifugation at 10,000 rpm for 60 min. at 4 ┬░C and by filtration through a 0.22 um filter (Corning). Supernatant was aliquoted and stored at 80 ┬░C until used.
Cultured cells were labeled with 3H-glucosamine (10 uCi/mL) for 24 hours. Supernatants were collected after microfuge for 5 minutes and transferred to new tubes. After adding 4 ╬╝L of 10% SDS and boiled for 3 min, samples were kept in ice and microfuge for 5 minutes. To the 50 ╬╝L of supernatant, 150 ╬╝L of sample buffer was added. Then, the sample was loaded over the sepharose CL-4B (Pharmacia) gel-filtration column chromatography (0.7├Ś50 cm) after passing through the running buffer through the column for more than an hour. Each fraction was collected for 3 minutes in a bottle. After adding the cocktail solution, the radioactivity (C.P.M) of the 3H-mucin was counted in the gamma-counter.
Total RNA was isolated from the cultured rat tracheal epithelial cells by using the Trizol reagent (GIBCO BRL). cDNA was synthesized from 5 ug of total RNA by adding 1 ╬╝L of random hexamer and enough DEPC water to bring the volume to 12 ╬╝L. The reactions were incubated at 25 ┬░C for 10 minutes and quick chilled on ice. After brief centrifuge, 4 ╬╝L of 5X first strand buffer, 2 ╬╝L of 0.1 M DTT, 1 ╬╝L of 10 mM dNTP mixture were added. After incubating the mixtures at 42 ┬░C for 2 min, 1 ╬╝L of (200 U) of Superscript II reverse transcriptase (GIBCO BRL) was added and incubated the mixtures for 50 minutes at 42 ┬░C.
PCR was performed by mixing the 5 ╬╝L of cDNA template, 45 ╬╝L of PCR supermix (GIBCOBRL), and 2 ╬╝L of each MUC5AC or GAPDH primer pairs (200 nM). The primer sequences for rat MUC5AC and GAPDH were as follows: MUC5AC, 5-GTTCTGAGATGTCCCTCCAC-3 5-GAATGGCCAAGCTTAGGCTG-3, GAPDH, 5-CGTC TT CA CC ACCATGGAGA-3 5-CGGCCATCACGCCA CAGTTT-3.
Cells were lysed in RIPA buffer containing phosphatase e inhibitor cocktail II and protease inhibitor cocktail (SIGMA). After harvesting the pellet, they were shaken for 30 minutes at orbital shaker at 4┬░C and the supernatants were collected after centrifuge (14,000 rpm) at 4┬░C for 10 minutes. Samples were mixed with an equal volume of sample buffer (2X) and boiled for 5 minutes. 30 ╬╝L of samples were loaded in the glass-plate sandwich (Mini-PROTEAN II; Bio-Rad) which contains 7.5% SDS-polyacrylamide gels. Power was applied to the Mini-PROTEAN II for 25 mAmp and electrophoresis begin. The gel was removed and transferred to the PVDF membrane for one hour. The PVDF membrane was blocked with blocking solution (5% skim milk) during overnight and rocked gently for 2 hours at room temperature after adding a phosphotyrosine monoclonal antibody (1:5,000 dilution). After washing, HRP-goat anti-mouse IgG (1:5,000 dilution) was added and rocked for one hour. The density in the PVDF membrane was developed by using the ECL detection reagents (Amersham) in the dark room.
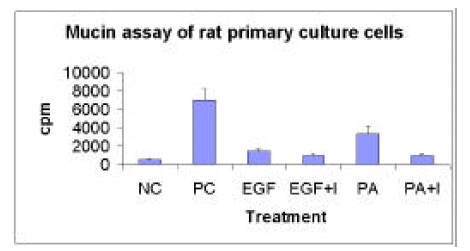
1. Mucin assay : the amounts of mucin secreted from the cultured rat tracheal epithelial cells were assayed after labeling 3H-glucosamine (10 uCi/mL) for 24 hours. EGF (50ug/mL) and Pseudomonas aeruginosa extract (1:40) significantly increased the mucin secretion and tyrphostin AG 1478 and selective EGF receptor tyrosine kinase inhibitor inhibit the mucin secretion from the epithelial cells (Figure 1).
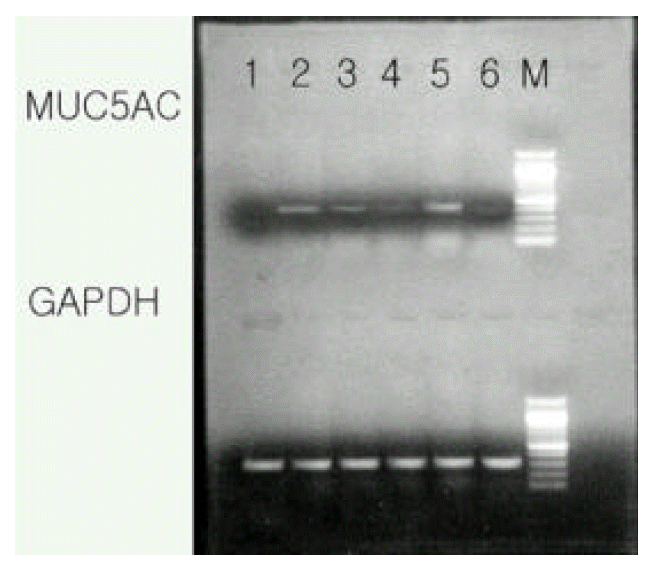
2. RT-PCR for MUC 5AC mRNA ; as with the mucin assay results, the EGF and Pseudomonas extracts increased the MUC5AC mRNA expression and the tyrphostin AG 1478 decreased the MUC5AC mRNA expression, respectively. Pseudomonas extracts more strongly increased the MUC5AC mRNA expression than from the EGF stimulation (Figure 2).
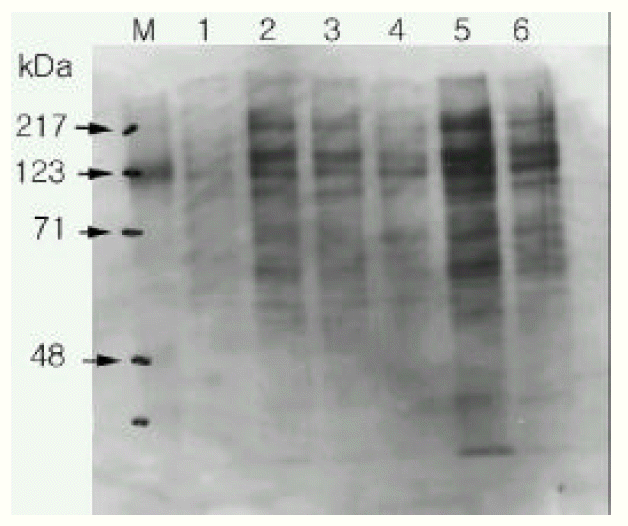
3. Western blot analysis of phosphotyrosine : we measured the effect of EGF and Pseudomonas extract to the EGFR tyrosine phosphorylation in the rat tracheal epithelial cells. EGF and Pseudomonas extract phosphorylated the tyrosine residue on the EGFR (about 180 KDa) and tyrphostin AG1478 nearly completely blocked the tyrosine phosphorylation (Figure 3).
Goblet cell hyperplasia is an important feature in many chronic airway diseases, including chronic bronchitis and bronchiectasis. In the tracheobronchial epithelium, mucins are synthesized by the goblet cells in the surface epithelium and mucous cells in the submucosal glands. Hypersecretion from hyperplastic goblet cells can cause obstructive airway disease and is reported as a major cause of death in acute severe asthma. Growth factors could be involved in goblet-cell production, because hypersecretory diseases are associated with abnormal epithelial-cell growth and proliferation. Among the growth factors, epidermal growth factor (EGF) and its stimulation of its receptor (EGF-R) is expressed on various cells. This EGF system is important in mucin secretion and MUC5AC gene expression in MUC5AC-inducing epithelial cell line, NCl-H2922). But these in vitro experiments did not confirm the importance of EGF system in primary epithelial cells, so we investigated whether EGF or Pseudomonas stimulate the EGF-R activation and mucin secretion by way of MUC5AC gene expression in rat tracheal epithelial cells. In our experiments, EGF induced EGF-R tyrosine phosphorylation, MUC5AC gene expression and mucin secretion in rat tracheal epithelial cells. EGF-R tyrosine kinase inhibitors AG1478 prevent not only EGF-R tyrosine phosphorylation but also mucin secretion and MUC5AC gene expression by EGF. Inhibitors of tyrosine kinase signaling cascade also attenuated the release of leucotriene and bronchial contractile response during antigen challenge in Guinea-pig4). Interestingly, serum has a strong capacity in the EGF-R phosphorylation, MUG5AC mRNA expression and mucin secretion when it was added in addition to the EGF (Lane 2 in the Figure 2, 3). So, it would be reasonable to use the bovine pituitary extract instead of serum in the study of mucin secretion from the rat tracheal epithelial cell culture27).
EGFR tyrosine phosphorylation leads to membrane phosphorylation which further leads to membrane-associated Ras activation, and downstream signaling to the nucleus5, 6). Tyrosine phosphorylation of EGF-R, a 180-Kda membrane glycoprotein, occurred in asthmatic airway in parallel with MUC5AC mRNA expression7). The EGF-induced EGF-R tyrosine phosphorylation was known to be due to the generation of hydrogen peroxide which was produced by EGF21). Activation of the EGF receptor signaling pathway occurred in human airway epithelial cells after stimulation with metals, IL-13 and asbestos8ŌĆō10). Of the mucin genes expressed in respiratory epithelium, MUC5AC appears to be one of the major respiratory mucins and MUC5AC glycoprotein is a major component of respiratory secretions from subjects with bronchial asthma11) and normal subjects12).
We measured the amount of secreted mucin from the rat tracheal epithelial cells after labeling 3H-glucosamine for 24 hours by chromatography over Sepharose CL-4B and measured the radioactivity. This method was laborious and time-consuming, so ELISA method is developed recently and is known to have an identical sensitivity as a gel-filtration assay13). A variety of mediators have been documented to up-regulate airway mucin secretion. These include (1) neurotransmitters released from cholinergic, adrenergic and nonadrenergic, noncholinergic nerve fibers; (2) lipid mediators, such as platelet-activating factor, leukotrienes and prostaglandins; (3) inflammatory cell products, such as histamine, elastase, cathepsin G, eosinophilic cationic protein, tumor necrosis factor-╬▒ and oxygen free radicals; (4) plasma-derived mediators, such as complement and bradykinin; (5) bacterial products, such as endotoxin, and proteinases22ŌĆō26).
It is well known that bacterial infection of the lung is associated with mucin overproduction. But, the link between infection and mucin overproduction is poorly understood. Recently It was found that Pseudomonas culture supernatant stimulates transcription of the MUC2 and MUC5AC gene in both bronchial explants and cultured airway epithelial cells14, 15). In our experiment and Pseudomonas culture supernatant markedly increased the mucin secretion and MUC5AC gene expression, EGF receptor tyrosine phosphorylation in the primary rat tracheal epithelial cell cultures. In spite of the dilution to 1:40 of the Pseudomonas culture supernatant, the response of the EGF receptor tyrosine phosphorylation, MUC5AC gene expression and mucin secretion was more strong than after EGF stimulation. In the case of MUC2 mucin gene, P.aeruginosa activates MUC2 mucin gene transcription by activation of a Src-dependent Ras-MEK1/2-SRK1/2-NF-kB pathway5).
Mucin genes are believed to be expressed during goblet cell growth20). Of the nine human genes that have been identified in the respiratory, gastrointestinal and reproductive tract, MUC5AC is the only mucin gene product isolated from normal human airway secretions and is therefore proposed to be a major airway secretory mucin1). Mucus hypersecretion can be induced experimentally by exposure of the rats to the respiratory tract irritants including tobacco smoke16) and acrolein17). The mechanism of MUC5AC gene activation by P.aeruginosa in primary epithelial cell culture is still unknown, but Pseudomonas exoproducts activate the transcription of MUC5AC, and the elements responsible for the activation of the reporter have been identified within 4 Kb of the transcriptional start site18). Neutrophil elastase increased the MUC5AC mRNA expression by increasing mRNA stability in A549 cells19).
In summary, we found that either EGF or Pseudomonas extracts phosphorylated the tyrosine residue in the EGF receptor from the rat tracheal epithelial cells, and this tyrosine phosphorylation was nearly completely blocked by selective EGF-R tyrosine kinase inhibitor, tyrphostin AG1478. The mucin secretion was also stimulated by either EGF or Pseudomonas extracts but more strong secretion of mucin and MUC5AC gene expression in the rat tracheal epithelial cell was done by Pseudomonas extracts. These mucin secretions and MUC5AC gene expression by Pseudomonas extracts were also suppressed by tyrphostin AG1478. Our data suggested that more extensive study about MUC5AC signal transduction pathway and the mechanism of MUC5AC gene over-expression by stimulation with P.aeruginosa in the primary tracheal epithelial cells might open up new targets for therapeutic intervention in the case of mucus hypersecretion by Pseudomonas lung infection.
Figure┬Ā1.
Mucin secretion from the cultured rat tracheal epithelial cells was measured by using the 3H-glucosamine (10 uCi/mL) and sepharose CL-4B gel-filtration column chromatography (0.7├Ś50 cm). The radioactivity of 3H-mucin was counted in the gamma counter. NC; negative control, no serum and no EGF medium (M/D+6F). PC; positive control, 5% serum and EGF (50 ng/mL) containing medium. EGF; EGF (50 ng/mL) in no serum medium. EGF+I; EGF (50 ng/mL) plus tyrphostin AG 1478 (10 uM) in no serum medium. PA; Pseudomonas extracts (1:40 dilution) in no serum medium. PA+I; Pseudomonas extracts (1:40) plus tyrphostin AG 1478 in no serum medium.
Figure┬Ā2.
MUC 5AC mRNA expression in the rat tracheal epithelial cells. Representative RT-PCR showed that both EGF and Pseudomonas stimulated the MUC 5AC mRNA expression and selective tyrosine kinase inhibitor typhorstin AG 1478 blocked the MUC 5AC mRNA expression. As was the case of mucin secretion, Pseudomonas markedly increased the MUC 5AC mRNA expression than from the EGF stimulation.
Figure┬Ā3.
Effects of EGF and Pseudomonas on the rat tracheal epithelial cell EGF-receptor tyrosine phosporylation. EGF and Pseudomonas increased the EGF phosphotyrosine band (180 Kda, arrow) and selective tyrosine kinase inhibitor tyrphostin AG 1478 completely inhibit the tyrosine phosphorylation.
Lane 1 : negative control, culture medium without EGF and without serum
Lane 2 : positive control, culture medium with EGF and with serum
Lane 3 : culture medium with EGF (25 ng/mL)
Lane 4 : EGF + typhorstin AG 1478
Lane 5 : Pseudomonas extracts (1:40)
Lane 6 : Pseudomonas extracts + typhorstin AG 1478
REFERENCES
1. Rose MC, Kaufman B, Martin BM. Proteolytic fragmentation and peptide mapping of human carboxyamidomethylated trracheobronchial mucin. J Biol Chem 264:8193ŌĆō81991989.

2. Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci 96:3081ŌĆō30861999.



4. Tsang F, Wong WSF. Inhibitors of tyrosine kinase signaling cascade attenuated antigen challenge of Guinea-pig airways in vitro. Am J Respir Crit Care Med 162:126ŌĆō1332000.


5. Li JD, Feng W, Gallup M, Kim JH, Gum J, Kim Y, Basbaum C. Activation of NF-kB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc Natl Acad Sci USA 95:5718ŌĆō57231998.



6. Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell 61:203. 1990.


7. Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med 163:511. 2001.


8. Wu W, Graves LM, Jaspers I, Devlin RB, Reed W, Samet JM. Activation of the EGF receptor signaling pathway in human airway epithelial cells exposed to metals. Am J Physiol 277:L924ŌĆōL9311999.


9. Zanella CL, Timblin CR, Cummins A, Jung M, Goldberg J, Raabe R, Tritton T, Mossman BT. Asbestos-induced phosphorylation of epidermal growth factor receptor is linked to c-fos and apoptosis. Am J Physiol 277:L684ŌĆōL6931999.


10. Shim JJ, Dabbagh K, Ueki IF, Dao-Pick T, Burgel PR, Takeyama K, Tarn DCW, Nadel JA. IL-13 induces mucin production by stimulating epidermal growth factor receptors and activating neutrophils. Am J Physiol 280:L134ŌĆōL1402000.

11. Meerzaman D, Charles P, Daskal E, Polymeropoulos MH, Martin BM, Rose MC. Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (MUC5AC). J Biol Chem 269:12932ŌĆō129391994.

12. Thornton DJ, Carstedt I, Howard M, Devine PL, Price MR, Sheehan JK. Respiratory mucins: identification of core proteins and glycoforms. Biochem J 316:967ŌĆō9751996.



13. Shin CY, Kang SJ, Kim KC, Ko KH. Comparison between ELISA and gel-filtration assay for the quantitation of airway mucins. Arch Pharm Res 21:253ŌĆō2591998.


14. Li JD, Dohrman A, Gallop M, Miyata S, Gum J, Kim Y, Nadel J, Prince A, Basbaum C. Transcriptional activation of mucin by P.aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA 94:967ŌĆō9721997.



15. Dohrman A, Miyata S, Gallup M, Li JD, Chapelin C, Coste A, Escudier E, Nadel J, Basbaum C. Mucin gene (MUC2 and MUC5AC) upregulation by gram-positive and gram-negative bacteria. Biochem Biophy Acta 1406:251ŌĆō2591998.

16. Coles SJ, Levine LR, Reid L. Hypersecretion of mucus glycoproteins in rat airways induced by tobacco smoke. Am J Pathol 94:459ŌĆō4721979.


17. Borchers MT, Wert SE, Leikauf GD. Acrolein-induced MUC5ac expression in rat airways. Am J Physiol 274(Lung Cell Mol Physiol 18):L573ŌĆōL5811998.


18. Li JD, Gallup M, Fan N, Szymkowski DE, Basbaum CB. Cloning the amino-terminal and 5ŌĆÖ-flanking region of the human MUC5AC mucin gene and the transcriptional up-regulation by bacterial exoproducts. J Biol Chem 273:6812ŌĆō68201998.


19. Voynow J, Young LR, Wang Y, Horger T, Rose M, Fischer BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol 276(Lung Cell Mol Physiol 20):L835ŌĆōL8431999.


20. Basbaum C, Lemjabbar H, Longphre M, Gensch E, McNamara N. Control of mucin transcription by diverse injury-induced signaling pathways. Am J Respir Crit Care Med 160:S44ŌĆōS481999.


21. Bae YS, Kang SW, Seo MS, Bainess IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. J Biol Chem 272:217ŌĆō2211997.


22. Larivee P, Levine SJ, Rieves RD, Shelhamer H. Airway inflammation and mucus hypersecretion. Airway secretion: Physiological Basis for the Control of Mucus Hypersecretion. In: Takishima T, Shimura S, eds. Marcel Dekker, New York: 469ŌĆō511.
23. Levine SJ, Larivee P, Logun C, Angus W, Ognibene FP, Shelhamer JH. Tumor necrosis factor-╬▒ induces mucin secretion and MUC-2 gene expression by human airway epithelial cells. Am J Respir Cell Mol Biol 12:196ŌĆō2041995.


24. Dwyer T, Farley JM. Human neutrophil elastase releases two pools of mucin-like glycoconjugate from submucosal gland cells. Am J Physiol Lung Cell Mol Physiol 278:L675ŌĆōL6822000.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



