INTRODUCTION
Helicobacter pylori (HP) has been strongly associated with peptic ulcers and it is thought to be a main etiologic factor in peptic ulcer disease1ŌĆō6). The eradication of HP prevents recurrent bleeding in bleeding peptic ulcers7ŌĆō15). Since several studies have reported that prevalence rate of HP infection may be underestimated in patients with bleeding peptic ulcers, it is debated that multiple tests or methods should be preferred for the diagnosis of HP in patients with bleeding peptic ulcer diseases9, 16ŌĆō19). Although various diagnostic tests are introduced with their own efficient outcomes, several limitations as diagnostic tests are reported in bleeding peptic ulcers because of intragastric blood and possibility of changed numbers of organisms by anti-ulcer medication19ŌĆō22). This study was designed to find out the best method for diagnosis of HP infection, in aspect of deciding the times of detection and the specific tests in bleeding peptic ulcers. We wanted to evaluate the validity that noninvasive test can execute a diagnostic role in bleeding peptic ulcers.
MATERIAL AND METHODS
Patients selection and characteristics
From September 1998 to July 1999, we prospectively tried tests two times (first 24 hrs and 7th day after initial therapeutic endoscopy) for 32 patients of bleeding peptic ulcers to detect HP infection by histologic study, rapid urease test (CLO test), carbon 13 labeled urea breath test (UBT) and serologic examination. We evaluated the sensitivity of each test, compared the two-times results and evaluated the effect to an outcome of endoscopic hemostasis.
We excluded the patients who has taken anti-ulcer medication or antibiotics or nonsteroidal antiinflammatory drugs (NSAIDs) before admission. Clinical features and endoscopic characteristics are recorded from each patient. Each patient or relative gave his or her written consent before the test and therapeutic endoscopy was performed. The test and treatment protocol had been approved by the Ethics Committee of the Soonchunhyang University Hospital (Chonan, Republic of Korea).
Diagnostic methods
During endoscopy, at first we performed endoscopic hemostasis by hemoclipping and/or hypertonic saline epinephrine (HSE) injection. After we confirmed initial hemostasis, we performed a multiple test for HP infection, including rapid urease test (CLO test, Ballard, Australia), which was observed for up to 24 hours, and histologic examination from endoscopic biopsy, 13C-labeled UBT and serologic examination for the presence of immunoglobin G (IgG) antibody to HP (Helicobacter pylori IgG, Radim, Italy).
After initial endoscopic hemostasis, a total of 4 biopsy specimens were obtained as two sets of antrum and body regardless of the presence of intragastric blood and one set of antrum and body was used for the CLO test and monitored for color change for up to 24 hours at room temperature. Another set was sent to an experienced pathologist for histologic examination with routine and special stains (modified Giemsa or Warthin-Starry silver stain).
After endoscopic hemostasis and aninvasive diagnostic test, we performed the 13C-UBT. The test consisted of a baseline breath sample and second breath sample collected 15 minutes after oral administration of 75 mg of 13C-labeled urea (HelikitŌäó, Isotechnica Inc, Canada) dissolved in tap water. If the value of the carbon dioxide expired in both samples differed by more than 4 per mil, this was considered a positive result.
The blood sample for serologic evaluation was obtained before any medication was begun. An enzyme-linked immunosorbent assay for IgG antibody to HP was performed with a cutoff of 30 UR/mL. It was suggested by the manufacturer.
Criteria for second study
Through the first-day study, the patients who hade two or more positive results among multiple tests (CLO test, histologic examination, UBT and serologic examination) were included. We did not exclude patients with rebleeding to evaluate the safety of the diagnostic test. Patients were carefully observed and given follow-up treatment through histamine-2 receptor antagonists (ranitidine, 50 mg intravenously every 6 hours), antacid and antihemorrhagic drugs. However, we avoided other medicine or procedure which affect HP concentration of the stomach. At 7th day after initial study, we repeated the multiple test, except the serologic examination, for HP infection through the same methods an the first-day study.
Statistical analysis
The sensitivity of each test for HP infection was calculated. To assess whether each test was statistically different in sensitivity between the two day studies, FisherŌĆÖs exact or Chi-square test was used. Chi-square test was performed to evaluate the difference in sensitivity between the CLO test and the 13C-UBT.
RESULTS
Sensitivity of diagnostic tests
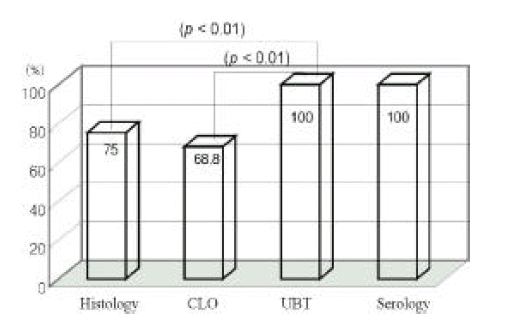
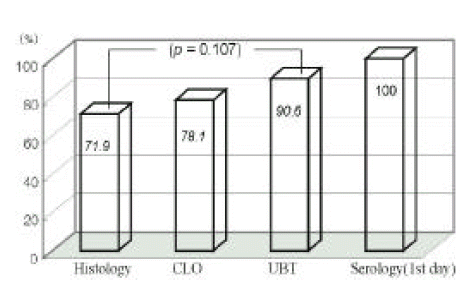
Diagnostic sensitivities of histologic study, CLO test, UBT and serologic examination were 75% (24/32), 68.8% (22/32), 100% (32/32), 100% (32/32) at first endoscopy, respectively, and those of the former three tests were 71.9% (23/32), 78.1% (25/32), 90.6% (29/32) at 7th day endoscopy.
Comparison of diagnostic test sensitivities
The sensitivities of the histologic study and CLO test were significantly lower than other tests (UBT or serologic examination) at first day study (p<0.01, Figure 1). Histologic, CLO, and UBT have limitations at 7th day endoscopy (Figure 2).
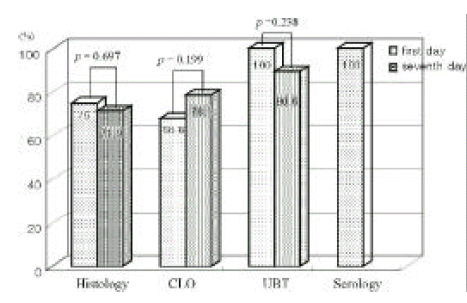
The sensitivities of the UBT had a tendency of decrease at examination of 7th day (p=0.238), and sensitivity of the CLO had a tendency of increment at examination of 7th day (p=0.199) (Figure 3).
The sensitivity of the diagnostic test according to the site of the lesion was not statistically different (Table 1).
As outcomes of endoscopic hemostatic treatment, initial hemostasis rate was 93.8% (30/32), rebleeding rate was 9.4% (3/32) and operation rate was 6.2% (2/32). 3 rebleeding patients were completely controlled by subsequent complete endoscopic hemostasis. All diagnostic tests at initial endoscopy did not influence the outcome of hemostasis compared to our previous result for hemostatic efficacy in bleeding peptic ulcers20).
DISCUSSION
There is still no established gold standard for the diagnosis of HP infection. The choice of diagnostic modality to determine HP infection status depends on test availability, ease of use, HP treatment history and whether esophagogastroduodenoscopy is planned. It has been debated which diagnostic test should be preferred for the diagnosis of HP in patients with peptic ulcer diseases. There are many presumable limitations of various diagnostic tests, which include changes of urease activity, changes of pH in sample or test medium, changes of number of HP organism, cost, time consumption, technical personal effort, clinical state of patients and patientŌĆÖs preference. Intragastric blood and possibility of changed numbers of organisms by medication have been known as representative problem to diagnose HP infection correctly in bleeding peptic ulcer. In our designed two-times test (first day and seventh day), intragastric blood, clinically emergent state and invasiveness of the test itself are suspected as limitary factors at first day; unavoidable medicine, including acid suppressive agent, antacid and antihemorrhagic drug, are suspected as limitary factors at seventh day.
There are few published studies that compare the sensitivity of the invasive and noninvasive diagnostic test. It is important to determine the best diagnostic method of HP infection, because it is possible that invasive test aggravates the ulcer bleeding in the emergent clinical state of peptic ulcer bleeding. Noninvasive tests have disputed that these are more useful than the invasive tests and more accurately reflect HP infection status24, 25). High frequency of positive results in noninvasive tests (13C-UBT and IgG serologic studies) was detected in our bleeding peptic ulcer patient in the first day, and UBT showed relatively high sensitivity at the seventh day. Sensitivity of the histologic test and CLO test was significantly low in the first day. In bleeding patients, we could not take more than four biopsy samples. Although more numbers of biopsies in extended site are expected to increase the result of sensitivity, we could not expand the design to take more biopsy samples in bleeding patients who were clinically unstable, in fact.
Since almost results of diagnostic tests exceed 97% of positive predictive value in peptic ulcer disease, repetitive tests are considered unnecessary in a patient who has one of the positive parameters for HP22). However, the prevalence rate of HP infection in patients with bleeding peptic ulcers has been reported to be lower than the rate in patients with nonbleeding ulcers in several studies, and our positive results of 68.8% CLO test and 75% of histologic test are low like the results of those studies19ŌĆō21). If we have to select one diagnostic test to detect HP infection in patients with bleeding peptic ulcers, we make important decision about which test is preferred to represent an the correct state of HP infection in bleeding peptic ulcers. There have been known factors that indicate false negative result of each diagnostic test and these include the bleeding and medication of antibiotics, H2-receptor antagonist or proton pump inhibitors, as well as limitation of each test itself.
Although UBT is a good diagnostic noninvasive test, the factor related to false negative results is not discussed in patients with bleeding peptic ulcers. Cut-off value and gastric emptying time are main factors that decide correct diagnostic yield26ŌĆō30). Intragastric blood and 7 days medication and gastric motility are suspected of false negative factors in the clinical setting of bleeding peptic ulcers. We may consider intragastric blood and changed gastric emptying time related to an unstable clinical state, and these can affect hydrolysis of labeled urea by urease producing HP, in contrast to unavoidable medication of H2 receptor blocker which can decrease the amount of urease itself. In our study, 100% positive result of UBT in the first day represents that intragastric blood and gastric motility of unstable clinical state are not a factor to change results, and 90.6% of 7 days can make us suspect the effect of medication.
CLO and histologic studies could not completely detect HP infection in two-times tests also. This means that these are not good diagnostic tests in patients with bleeding peptic ulcers.
Serological testing has been well-known for easy performance and high positive result, but it also has limitations because interpretation of its results is difficult to differentiate past infection in countries with a high HP prevalence rate. Therefore, it is usually used in a limited setting of follow-up studies after HP treatment.
In conclusion, first-day histologic and CLO test are an inadequate method in detecting HP infection in patients with bleeding peptic ulcers. 7-day histologic, CLO test and UBT have a low sensivity. Intragastric blood at first day and anti-ulcer medications during 7-days are suspected as risk factors of decreased sensitivity. First-day UBT can be a standard test to diagnose HP infection in patients with bleeding peptic ulcers.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





