INTRODUCTION
Cytomegalovirus (CMV) disease is a major cause of morbidity and mortality in immunocompromised patients, particularly recipients of solid organ or bone marrow allograft, and patients with the acquired immune deficiency syndrome1). Although CMV pneumonia is well recognized as the commonest infectious cause of death in bone marrow allograft recipients, the morbidity associated with CMV gastroenteritis is sometimes overlooked. while chemoradiation treatment, graft-versus-host disease and various medications may lead to nausea and vomiting following bone marrow transplantation(BMT), persistent symptoms cannot be explained in many cases and some cases may represent unrecognized gastrointestinal(GI) infections2). Cytomegalovirus is the most prevalent infectious agent in the upper GI tract of symptomatic BMT recipients. Before the introduction of effective antiviral therapy, mortality of CMV infection was very high, but the use of ganciclovir has improved the prognosis. However, the appropriate method for treatment of CMV-associated enteritis is still unknown.
We report here a case of CMV duodenitis which developed following allogenic BMT and was successfully treated with ganciclovir.
CASE REPORT
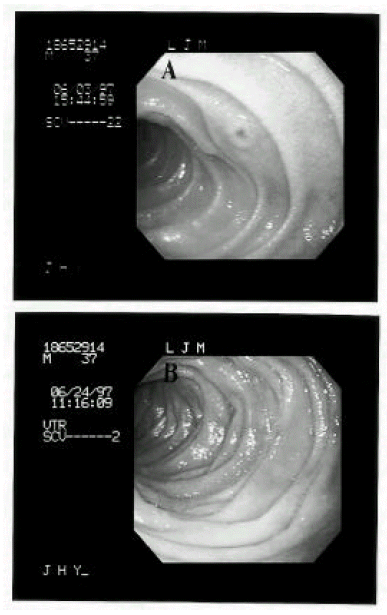
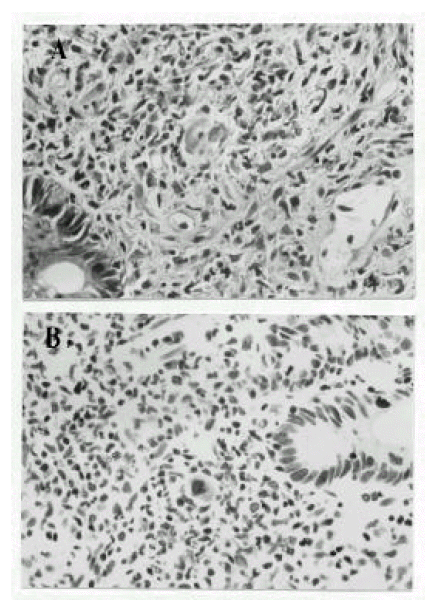
A 38-year-old male was diagnosed as acute lymphoblastic leukemia in December 1996. He received induction chemotherapy consisting of daunorubicin, vincristine, prednisolone and L-asparaginase. After two cycles of induction chemotherapy, complete remission was achieved and allogenic BMT was performed. Preparative chemotherapy for BMT consisted of busulfan (1mg/kg p.o every 6 hours for 4 days) and cytoxan (60mg/kg iv for 2 days). He received a bone marrow graft from a HLA matched sibling donor. Serologic tests for IgG CMV antibody were positive in both recipient and donor. For GVHD prophylaxis, cyclosporin and a short course of methotrexate were administrated. Granulocyte colony-stimulating factor (450 μg/day) was given beginning on day 5. Enteral decontamination was done with ciprofloxacin and acyclovir was given for the prophylaxis of viral infection. CMV blood culture was performed weekly until 100 days post-BMT. The early post-BMT course was uneventful and the patient was considered to achieve engraftment by bone marrow aspiration and biopsy on post-transplant day 21. He had, however, prolonged nausea and vomiting for 5 weeks after BMT and mild fever and watery diarrhea was developed, all of which necessitated hyperalimentation persistently. Physical examination revealed no abnormalities except mild epigastric tenderness. Laboratory values included a WBC 4,200/mm3, hemoglobin 9.0 mg/dL, hematocrit 25.7%, platelet 34,000/mm3, bilirubin, liver transaminase, BUN and creatinine were normal and the stool was negative for C. difficile toxin. Weekly CMV blood cultures were all negative. For the evaluation of the prolonged nausea, vomiting and diarrhea, gastrofibroscopy and flexible sigmoidoscopy were performed on post-transplant day 39. By gastroscopic finding, the stomach showed multiple hyperemic patchy erosions mainly in the greater curvature of the body and, on the second portion of the duodenum, an octopus sucker-like elevated lesion with central hyperemic erosion and several hyperemic flat erosive lesions were noted(Fig. 1-A). Sigmoidoscopic finding was normal. Gastric mucosal biopsy showed mild gastritis. Duodenal mucosal biopsy revealed cytomegalic cells which showed a large, densely staining nucleus and abundant cytoplasms with intracytoplasmic inclusion body(Fig. 2-A). Immunochemical staining for CMV antigens using monoclonal antibodies confirmed the diagnosis of CMV duodenitis(Fig. 2-B). The patient was treated with ganciclovir (5 mg/kg iv, twice a day for 3 weeks) for the CMV duodenitis. Nausea and vomiting were decreased and total parenteral nutrition could be stopped. At the end of 3 week’s treatment, follow-up gastrofibroscopy revealed healed erosions(Fig. 1-B). Maintenance treatment with ganciclovir was not given. He was free of CMV infection until 288 days after allogenic BMT.
DISCUSSION
The gastrointestinal disorders which are seen between day 0 and 20 post-transplant are generally related to toxicity of conditioning therapy, bacterial and fungal infection and side effects of drugs. Between day 20 to 30 post-transplant, the marrow graft becomes functional, but there is incomplete immunologic reconstitution. The abdominal symptoms at this time are commonly related to acute GVHD or viral infections, although venoocclusive disease may persist as well3). Thus, endoscopy with biopsy in symptomatic patients is an essential method for a definite diagnosis.
Conditions that specifically predispose to CMV disease of the GI tract in BMT recipients have not been identified, but presumably are similar to those identified as predisposing to CMV infection of any type. The relationship between GI GVHD and GI CMV infection or disease is not well understood. Both of these conditions commonly coexist following BMT. Whether GVHD of the gut may predispose to secondary invasion with CMV is not proven4,5). In our patient, we could not find evidence of GVHD.
An indication of the incidence of GI CMV disease was provided in a study by Spencer et al2), in which they performed upper GI endoscopy to investigate unexplained nausea and vomiting in BMT recipients. Nineteen(38 %) of the 50 patients had CMV infections of the esophagus and/or small intestine. In another study, 3 (12.5 %) of 24 BMT recipients had GI involvement with CMV at autopsy.
All levels of the GI tract have been reported as the sites of CMV infection. BMT recipients have a much higher likelihood of having involvement of the upper GI tract (esophagus, stomach and duodenum) than the lower GI tract6). However studies that systematically examine the entire GI tract of these patient populations are not available. Einsele et al7) reported that, because of the preferential involvement of the ascending colon and terminal ileum by CMV virus, a colonoscopy of the entire colon and terminal ileum, including biopsies from these sites, should be performed in all patients with severe diarrhea following allogenic BMT. Since endoscopic findings are variable, such as erythema, erosions, ulcerations, hemorrhage, nodules and polyps8), and radiological findings were nonspecific for CMV infection9), a biopsy was required for a definite diagnosis. The presence of cytomegalic cells on mucosal biopsy specimens stained with hematoxylin and eosin has been considered as the gold standard for establishing a diagnosis of CMV GI disease. To enhance the sensitivity and specificity of histopathologic analysis immunohistochemical staining methods for CMV antigens, using monoclonal antibodies, have been developed and utilized effectively. In our patient, we could be convinced of the diagnosis because the immunohistochemical staining confirmed the presence of CMV antigen in the biopsy specimen.
Although ganciclovir, an antiviral agent with both in-vitro and in-vivo activity against CMV, has been used in treatment of CMV disease10), the appropriate method for treatment of CMV enteritis is currently unknown, and therapy with ganciclovir has been less successful in the treatment of GI CMV disease in the BMT population than in the solid organ transplantation population. A placebo-controlled trial by Reed et al6) found no evidence of clinical or endoscopic improvement of GI CMV disease after 2 weeks of treatment with ganciclovir as compared with supportive care. However, on the basis of experiences in CMV pneumonitis, it is common practice to use ganciclovir. Immune globulin combined with ganciclovir may be helpful as in the CMV pneumonitis. The usual induction dose is 10 to 15 mg/kg per day administered in 2 to 3 divided doses daily for 3 weeks. Ganciclovir therapy for 3 weeks resulted in both a clinical and endoscopic remission of CMV duodenitis in our patient. It is not established whether maintenance therapy with ganciclovir should be given. In patients with severe and persistent immune deficiency, such as GVHD or AIDS, the relapse rate is high and some physicians advise maintenance therapy after induction therapy to prevent or delay relapse11). Our patient had no evidence of GVHD and his blood count recovered uneventfully. So, we decided not to give maintenance ganciclovir therapy and he was free of CMV infection post-transplant day 288.
In conclusion, CMV disease should be suspected in the BMT patients complaining of prolonged nausea and vomiting, and appropriate biopsies and cultures should be done to confirm the diagnosis. Prompt treatment with ganciclovir for the confirmed GI CMV disease may be helpful.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





