 |
 |
| Korean J Intern Med > Volume 13(2); 1998 > Article |
|
Abstract
CMV infection may occur anywhere in the gastrointestinal tract. Among the small intestine, ileum is the most common site of CMV disease and infection of jejunum is a rare one in patients with CMV gastroenteritis. Although rare, the reason why the recognition of this diagnosis is important is that it cause the lethal hemorrhage and perforation of gastrointestinal tract when its diagnosis and treatment was delayed. Rapid diagnosis are able to using the immunohistochemical stain in shell vial culture of infected specimen or peripheral neutrophils preparation in viremic patients within 8 to 36 hours. The treatment of choice is antiviral agent or surgical resection.
We experienced a case of CMV disease of jejunum in patient with non-Hodgkin’s lymphoma who showed severe ulceration in jejunum and massive intestinal hemorrhage, and he survived after successful treatment with segmental resection of jejunum and intravenous ganciclovir.
Cytomegalovirus(CMV) infection is widely seen in humans, from new-born to adults, being ubiquitously present and causes subclinical disease, mostly primary CMV infection may result in a mononucleosis-like syndrome in adolescents and young adults1). Isolated self-limited CMV colitis has been described in immunocompetent individuals2).
CMV infection and disease occur with high frequency in immunocompromised patients as an opportunistic infection. The CMV disease of the gastrointestinal tract in immunocompromised host may result in life-threating manifestations, such as hemorrhage, perforation and peritonitis3). With the exception of CMV colitis, CMV disease of small intestine, especially jejunal involvement, is a rare one and there was no case report in Korea. We report a patient of non-Hodgkin’s lymphoma who had CMV infection of esophagus, stomach and jejunum which was confirmed by CMV immunohistochemical stain of surgical specimen of jejunum and its culture.
A 43-year-old man was admitted to the our hospital because of relapsed non-Hodgkin’s lymphoma. Since three years ago, he had been treated with 8th cycles of ProMACE-CytaBOM chemotherapy for diffuse mixed small and large cell type malignant lymphoma and achieved complete remission. The disease relapse was noted 27 months later and he underwent salvage chemotherapy with CDME regimen(Cisplatin, Dexamethasone, Mitoxantrone, Etoposide). On the fifth day after chemotherapy, he developed fever and diarrhea and was diagnosed as fungal sepsis for Candida tropicalis and was treated with intravenous amphotericin B administration. On the following day, he developed sudden onset of abdominal pain and massive intestinal bleeding. On physical examination, breathing sounds were normal and abdomen was distended and tympanic with infrequent bowel sound. There was diffuse and mild tenderness on abdomen without rebound tenderness or muscle guarding. A rectal examination revealed melena. Laboratory finding revealed WBC 4,000/mm3 (neutrophils 84.7%, lymphocytes 5.0%, monocytes 9.8%), hemoglobin 9.1 g/dL, platelet 173,000/mm3. Blood and urine cultures showed no growth of bacteria and gastrointestinal bleeding was persisting. On the twenty-fifth hospital day, we performed gastrofiberscopy and total colonoscopy. On endoscopic findings, the discrete ulcerations, white patches and multiple erythema of distal esophagus and lesser curvature of stomach body portion were found, active bleeding is not noted. Endoscopic biopsy were performed in ulcer base and margin of esophagus and stomach. On total colonoscopic findings, there were only old blood clots in large bowel. 99mTc-RBC abdominal scan showed accumulation of radionuclide in left lower quadrant on 24 hours delayed scaning. It suggested bleeding from small intestine. He underwent the explorating laparatomy.
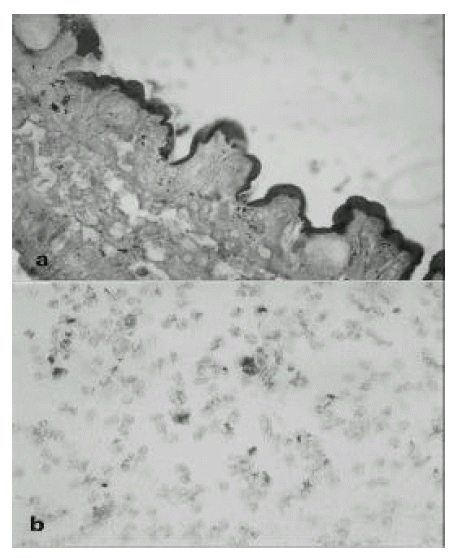
In operation fields, jejunal segment of small intestine showed a diffuse inflammatory process. There were dark blood clots in distal jejunum and ileum. The involved area of the small intestine was removed and the intestinal continuity was reestablished by end to end anastomosing the upper jejunum to the terminal ileum. The resection specimen consisted of 48-cm-long portion of jejunum. Grossly, there were multiple ill-defined ulcerations surrounded by irregular nodularities of mucosa(Fig 1 a, b). On histologic examination, the ulcer base was consisted of necrotic exudate and granulation tissue. Also, on HE stain, there was many enlarged cells with purple intranuclear inclusions surrounded by a clear halo and smaller basophilic cytoplasmic inclusion mainly in the submucosa and muscle layer of ulcerative areas. It suggested the cytomegalovirus infection of jejunum(Fig 2 a). On histologic examination of endoscopic biopsy specimen of esophagus and stomach, there were small basophilic inclusion bodies in endothelial cells and glandular cells(Fig 2 b). In our case, we performed the immunohistochemical staining to detect pp65 antigen of CMV in cytospin preparations of peripheral blood neutrophils(Fig 3 a) and paraffin-embedded surgical specimen of jejunum by the APAAP(alkaline phosphatase antialkaline phosphatase)(Clonal CMVKit, Biotest, Germany) method(Fig 3 b). Also, IF stain using DAKO-CMV, DDG9 and CCH2(DAKO, USA) for immediate early CMV antigen was performed from urine shell vial culture which showed heavy CMV infection.
After surgical resection of jejunum, he treated with gancyclovir for three weeks. He was improved and GI bleeding had stopped in post operation 1 day and has been followed at the out-patient clinic.
CMV infection may occur anywhere in the gastrointestinal tract and the most common sites of involvement of CMV are the colon, stomach and small intestine in decreasing order4). The ileum is the most common site of CMV infection among the small intestine but simultaneously jejunal involvement is rare. Kim et al5) reported the systemic CMV disease including colon and small intestine.
As the presenting symptoms, there are quite variable symptoms as generalized infection or localized GI tract infection6,7). CMV disease of the GI tract typically produces mucosal ulceration that can result in pain, bleeding, diarrhea and GI perforation, among which gastrointestinal bleeding was the most common presentation4,8).
The pathogenesis of CMV-associated ulceration is controversial. In Meiselman’s histopatholgic reports9), he suggested that the CMV may cause the ulceration in colon and that ulcerations arise from ischemic damaged due to CMV infection of small vessels in the mucosa and submucosa. Other authors10) have proposed that the virus may have an affinity for sites of preexisting ulceration and may not initiate the ulceration. CMV inclusions in the capillaries and venules of the mucosa and submucosa are believed to produce focal ischemias that are responsible for mucosal ulcerations. In Kazuki’s report11), perforation of jejunum associated with CMV infection occur in which involved by lymphoma were established in histopatholgy. In our case, although the involvement of non-Hodgkin’s lymphoma in jejunum was not established, we thought that the mucosal ulceration may occurs after chemotherapy or Candida infection of small intestine that the CMV infection was occurs at the ulceration site.
On diagnosis of CMV infection, tissue can be examined for the presence of characteristic CMV inclusion bodies using conventional CMV culture techniques with examination for a cytopathic effect, however, it take a long time. Also, simple seropositivity may be coincidental and may not indicate that the current clinical illness is being caused by CMV12). Rapid diagnosis of CMV infection has gained its importance as the number of individuals susceptible to clinical disease rises due to increased disease-related and iatrogenic immunosuppression especially associated with HIV infection, cancer chemotherapy and transplantation, and as therapeutic options neccessitating early diagnosis become available. The so-called shell vial culture techinique is one means by which the virus can be rapidly identified. The fibroblast monolayer is infected with culture specimen with centrifugal enhancement and stained with an immunofluorescent labeled anti- CMV- specific murine monoclonal antibody13). This assay is as sensitive as routine cultures and provides data within a relatively short period of time. Recently, direct viral antigen detection in buffy-coat preparations has been developed12). They have shown a correlation between detection of viral antigenemia and some CMV-associated symptoms. However, other investigators have not been able to reproduce these results14). Amplification of CMV-DNA presence within a specimen also can take place using the polymerase chain reaction technique, in which multiple copies of the viral DNA are amplicated in vitro and identified using a complementary CMV-DNA probe with either a radiolabel or an enzymatic marker that allows quantitation15).
The treatment of CMV disease has been advanced with the antiviral agent ganciclovir.
This drug clearly benefits solid organ transplant recipients who develop CMV infections and has proven effective for patients with CMV disease of gastrointestinal tract16,17). This drug should be used for the treatment of documented CMV disease of the GI tract in all groups of immunocompromised patients. Although relapses after ganciclovir therapy for CMV disease of the GI tract occur in up to 40% of immunocompromised patients, no data are available to recommend routine use of maintenance therapy18).
In this case, we found, only the nonspecific ulcerations in endoscopic finding, thus we can start the treatment when the CMV inclusion body was established in esophagus and stomach biopsy specimens. Although the immunohistochemical stain for surgical specimen of jejunum and urine culture in fibroblast were showed, we thought that the more rapid diagnostic technique, in use of immunohistochemical stain of shell vial culture of urine or peripheral neutrophils preparation, has must be preferrable.
Fig. 1.
Surgical specimen of jejunum a: Several discrete ulcers, irregular in shape and size, are seen. Intervening mucosa showed irregularity, nodularity, and pseudopolyp appearance, b: The ulcer base consist of necrotic exudate.
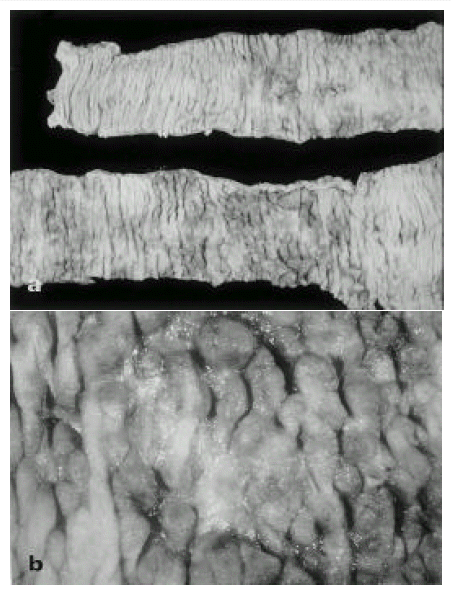
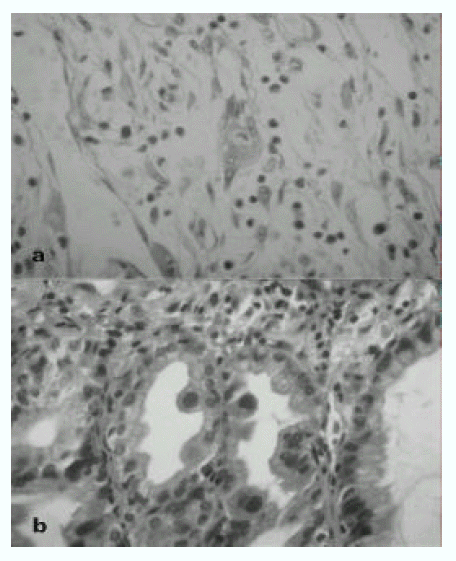
Fig. 2.
a: Cytomegalovirus inclusions in endothelial cells of jejunum. There are many enlarged cells with purple cytoplasmic inclusions in submucosa and muscle layer of ulceration area(H&E stain ×400). b: CMV inclusion body in glandular cells of endoscopic biopsy specimen of stomach(H&E stain ×400).
REFERENCES
1. John AZ. Epidemiology and pathogenesis of cytomegalovirus disease. Semin Hematol 1990;27(1):5–10.

2. Christina MS, David M. Self-limited cytomegalovirus colitis in immunocompetent individuals. Gastroenterology 1988;94:194–9.


3. Knapp AB, Horts DA, Eliopoulos G, Gramm HF, Gaber LW, Falchuk KR, Falchuk ZM, Trey C. Widespread cytomeglovirus gastroenterocolitis in patient with acquired immunodeficiency syndrome. Gastroenterology 1983;85:1399–1402.


4. Frederick SB, Claire P. Cytomegalovirus disease of the gastrointestinal tract in patients without AIDS. Clin Infect Dis 1993;17:644–56.


5. Kim JS, Lee DH, Lee JS, Lee KL, Kim YT, Yoon YB, Song IS, Choi KW, Kim CY. A case of cytomegalovirus colitis. Korean J Gastrointest Endosc 1991;12:107–113.
6. Song SY, Chon CY, Park YN, Lee SI, Lee HC, Kang JK, Park IS, Choi HJ, Park CI. Cytomeglaovirus (CMV)-associated gastric ulcer proved by dot-blot hybridization. Korean J Gastrointest Endosc 1992;12:237–244.
7. Hinnant KL, Rotterdam HZ, Bell ET. Cytomegalovirus infection of the alimentary tract: a clinicopathological correlation. Am J Gastroenterol 1986;81:944–50.

8. Cheung Annie NY, Irene OL. Cytomegalovirus infection of gastrointestinal tract in non-AIDS patients. Am J Gastroenterol 1993;88:1882–1886.

9. Meiselman MC, Cello JP, Margaretten W. Cytomegalovirus colitis. Report of the clinical, endoscopic and pathologic findings in two patients with the AIDS. Gastoenterology 1985;88:171–175.

10. Goodman ZD, Boitnott JK, Yardley JH. Perforation of the colon associated with CMV infection. Dig Dis Sci 1979;24:376–380.


11. Kazuki N, Ei S, Shohei I, Yoshito E, Yoichi M, Masashi K. Jejunal perforation associated with cytomeglaovirus infection in a patient with adult T-cell leukemialymphoma. Acta Pathol Jpn 1992;42:267–271.

12. The TH, van der Bij W, van der AP. Cytomegalovirus antigenemia. Rev Infect Dis 1990;12(7):S737–S744.

13. Paya CV, Smith TF, Hermans PE. Rapid shell vial culture and tissue histology compared with serology for the rapid diagnosis of cytomegalovirus infection in liver transplantation. Mayo Clin Proc 1989;64:670–675.


14. Miker H, Rossier E, Milk R, Thomas C. Prospective study of cytomegalovirus antigenemia in allograft recipients. J Clin Microbiol 1991;29:1054–1055.



15. Jiwa NM, Gemert GWV, Raap AK. Rapid detection of human cytomegalovirus DNA in peripheral blood leukocyte of viremic transplant recipients by polymerase chain reaction. Transplantation 1989;48:72–76.


16. Kaplan CS, Peterson EA, Icenogle TB. Gastrointestinal cytomegalovirus infection in heart and heart lung transplantation. Arch Intern Med 1989;149:2095–2100.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



