INTRODUCTION
ASA and non-steroidal anti-inflammatory drugs(NSAIDs) can induce bronchoconstriction in 10ŌĆō20% of adult asthmatic patients1,2). The mechanism of ASA-induced bronchoconstriction is partly clarified. Appreciable numbers of aspirinsensitive asthma had rhino-sinusitis and/or nasal polyp3ŌĆō6). Immunohistochemical study7) of the bronchial tissue from ASA-BA showed increased inflammatory cell infiltrations, including eosinophil, mast cell and lymphocyte, similarly to non-aspirin sensitive asthma. In this study, in order to further understand the pathogenic mechanism of ASA-BA, we tried to detect C3-containing IgG- and IgA-IC level in sera from ASA-BA patients and analyzed them based upon various clinical characteristics seen in ASA-BA.
METHODS
Subjects
Thirty three ASA-BA patients, aged between 23 and 72 years, participated in this study. All showed positive responses on lysine-aspirin (L-ASA) bronchoprovocation test (BPT), and they were compared with 14 allergic asthma patients sensitive to house dust mite, 14 intrinsic asthma patients and seven healthy controls. Their sera were collected and stored at ŌłÆ20┬░C. Atopy was defined on the basis that they showed more that 2+ skin prick test reactions to more than two common inhalant allergens, such as Dermatophagoides farinae, alder, oak, rye grass, mugwort, ragweed, Aspergillus spp. (Bencard Allergy Service, Bredford, Middlesex, UK).
L-ASA bronchoprovocation test
L-ASA BPT was performed according to the method with some modifications8ŌĆō10). Pulmonary functions were measured with a spirometer (Chest), the FEV1 and maximum midexpiratory flow (MMEF), before and during the provocation. The test solution was delivered by a De Vilbiss 646 nebulizer (DeVilbiss Co., Somerset, Penn., USA) and connected to a compressed air source (5 1/min). Normal saline was inhaled as a placebo solution. The patients were asked to breathe the nebulized aerosol up to their vital capacity, 10 times L-ASA (Young Jin Pharmaceutical Co. Korea), as a powder containing 1800 mg L-ASA was made up freshly on each challenge day. The challenges with the placebo were performed seven days before the L-ASA BPT. L-ASA BPT from 11.25 up to 180 mg/ml was performed to induce more than a 20% fall of FEV1. The FEV1 and MMEF were measured frequently during the first hour, and then hourly for 8 hours.
Measurement of C3-containing IgG- and IgA immune complex: (IC)
C3 containing IgG-IC was measured according to previously described methods with some modifications11ŌĆō13). A 96-well microplate (Dynatech, Alexandria, Va, USA) in 0.05M carbonate buffer (pH 9.6) at 4┬░C was used. After washing with phosphate-buffered saline with 0.05% Tween 20(PBST), the wells were blocked with 1% bovine serum albumin-PBST. The wells were then incubated with undiluted sera overnight at 4┬░C. After washes with PBST, they were incubated with 50 ╬╝l biotin-conjugated anti-IgG or anti-IgA antibodies (Vector laboratories, Burlingame, CA, USA) at a dilution of 1: 1000 (w/v) for 1 hour. The washing step was repeated, and streptavidin-conjugated peroxidase (Sigma, St. Louis, MO, USA) at a dilution of 1:1000 w/v was added into the wells and incubated for 30 minutes. After another wash, 50 ╬╝l of 0.01M ABTS (2,2ŌĆ▓-azino bis-3 ethyl-benzthiazoline sulfonic acid) in 0.07M citrate-phosphate buffer, pH 4.2 containing 0.03% H2O2 was added to the wells. The color reaction was stopped with 0.002M sodium azide and the plate was read at 410 nm using a microtiter reader (Dynatech Laboratories, Virginia, USA). The standard for quantification of C3-IgG- or IgA-IC was established by pooling systemic lupus erythematous patients sera with high IgG-IC levels, and arbitrarily defining the standard as 100 AU (arbitrary unit)/ml. Optical density of the test sera was compared to that from serially diluted standard and presented as AU/ml.
RESULTS
This study demonstrated IgG-IC and IgA-IC levels in three asthmatic groups and seven healthy controls as shown in Fig. 1. IgG-IC levels tended to be higher than those of IgA-IC. There was no significant difference in the IgG-IC level among the four groups (p>0.05). However, IgA-IC level was significantly higher in ASA-BA than in other groups (p=0.0035).
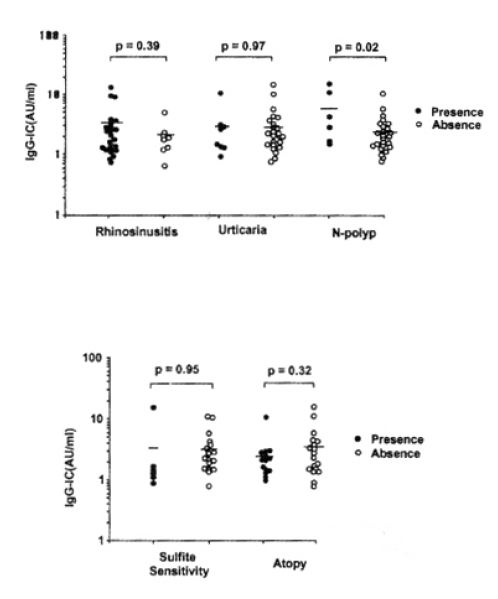
IgG-IC level was compared according to the presence of rhino-sinusitis, urticaria or nasal polyp. No significant difference was found in IgG-IC level, whether the patients have rhino-sinusitis or urticaria (p=0.39, p=0.97, respectively). However, the patients having nasal polyp had significantly higher IgG-IC level than those without it (p=0.02). No significant difference was found between IgG-IC level and atopic status or concurrent sensitivity to sulfite (p=0.32 p=0.95, respectively) as shown in Fig. 2.
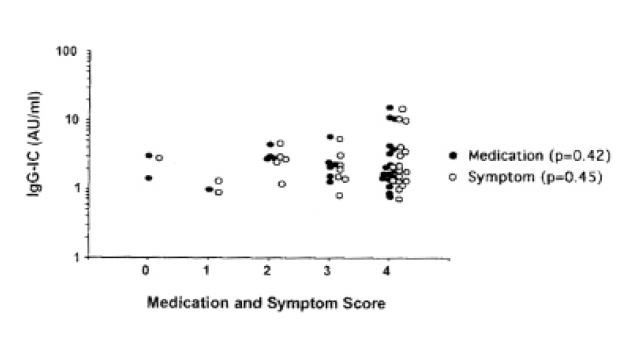
Fig. 3 shows the distribution of IgG-IC level according to medication and symptom scores. Most patients had high symptoms and medication scores (Class 3 or 4). No significant difference was found among the four groups (p>0.05).
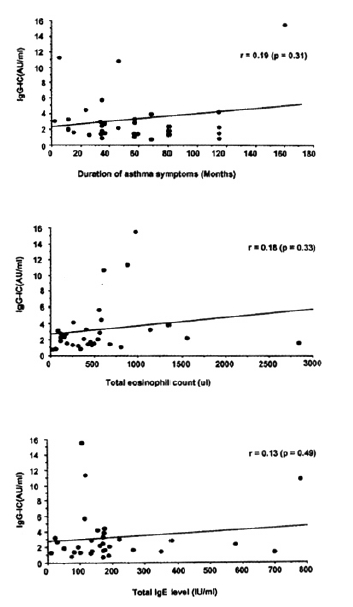
Fig. 4 shows the relationship between IgG-IC level and duration of asthma symptoms, total IgE level or eosinophil count. The correlation was insignificant with asthma symptom duration (r=0.19, p=0.31), total IgE level (r=0.13, p=0.49), or total eosinophil count (r=0.18, p=0.33)
DISCUSSION
The pathogenic significance of CIC in allergic diseases is unclear. Several investigators have reported IC, especially IgE-IC, increased in patients with allergic asthma and food allergy, especially after the food challenge test14ŌĆō16). In food allergy patients, IgE IC could activate the complement system and bind to the conglutinin column after the addition of fresh human sera, suggesting a participation of C3 in IgE-IC. Our previous study13) showed that C3-containing IgE-IC was more elevated in house dust mite-sensitive asthma than in non-allergic asthma. There have been some in vitro data supporting the possible involvement of IC in allergic inflammation. IC containing IgE could activate inflammatory cells, including monocyte, neutrophil17), and eosinophil18). Preformed allergen-IgE complex could induce immediate erythema and wheal reaction19). Moreover, activated C3 fragment could degranulate mast cell and activate monocyte, neutrophil and eosinophil20). Furthermore, there has been a report suggesting that an IC-mediated mechanism may contribute to late skin reaction on the basis that late reaction to the house dust mite on intradermal test could be accentuated when autologous serum was mixed with the allergen in sensitive individuals21). These results might suggest a possible involvement of C3-containing IC in the pathogenesis of atopic asthma. However, there have been very few reports to study the role of circulating immune complex in patients with ASA-BA. In the present study, we tried to detect C3-containing IgG- and IgA-IC levels in ASA-BA, and compared them with allergic and non-allergic asthma, as well as healthy controls. IgG-IC level was higher than IgA-IC in each group. No significant difference was noted in IgG-IC level among the four groups but, interestingly, IgA-IC was significantly higher in ASA-BA than in other groups. Further studies will be needed to identify the role of IgA-IC in airway inflammation of ASA-BA.
The definitive diagnostic test for respiratory sensitivity to ASA has been oral provocation with ASA and NSAIDs. L-ASA BPT has become an alternative diagnostic test to detect ASA sensitivity in asthmatic patients8,9,22). In our previous study10), L-ASA inhalation induced late asthmatic response as well as early reaction. The mechanism to induce late asthmatic response following L-ASA inhalation has been unknown. In the present study, 15 had early asthmatic response and 18 had a late onset asthmatic response (five dual and 13 late only). No difference was found in the IgG-IC level according to the type of asthmatic response following L-ASA inhalation. These results support that L-ASA BPT is a useful method to determine lower respiratory sensitivity to ASA, and IgG-IC may not be involved in the development of the late asthmatic response.
ASA-BA has a wide clinical spectrum23). High incidence of rhino-sinusitis and/or nasal polyp was reported in ASA-BA5,6,24). The frequency of polyp formation in patients with SamterŌĆÖs syndrome has ranged from 50 to 95 percent. Our recent investigation(unpublished data) on immunohisto chemical analysis of nasal polyp from ASA-BA revealed that the number of mast cell and eosinophil tended to be higher than non-allergic polyp, although the statistical significance was not reached. In this study, the patients with nasal polyp had significantly higher IgG-IC level than those without it. These findings may support a possible involvement of IgG-IC in the nasal polyp formation in ASA-BA. Further investigations will be needed to detect these ICs within the nasal polyp tissue.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





