INTRODUCTION
Mature peripheral T lymphocytes generally undergo cell death when stimulated with monoclonal antibodies against CD3/TCR complex and phytohemagglutinin (PHA)1). This process is rapid and exhibits classic characteristics of apoptosis, such as membrane blebbing, chromatin condensation and the formation of DNA fragmentations of -200bp. Deletion of T lymphocytes by apoptosis appears to be important not only in regulating autoreactive T lymphocytes in the thymus, but also in regulating the peripheral T lymphocytes pool2,3).
Fas antigen is a type I membrane protein belonging to the tumor necrosis factor (TNF)/nerve growth factor (NGF) receptor family4). Crosslinking with antibody against human Fas antigen induces apoptosis of cells expressing Fas antigen. From these results, it was concluded that Fas antigen transduces an apoptotic signal into cells, and that anti-Fas antibody works as an agonist for the Fas antigen.
In vitro and in vivo results suggest the involvement of the Fas system in the clonal deletion of autoreactive T lymphocytes in the periphery5). Mutation in the Fas-encoding gene is responsible for the lymphoproliferative disorder and associated lupuslike syndrome in Ipr and Iprcg mice6). Defects in Fas-induced apoptosis lead to the incomplete elimination of peripheral autoreactive cells in these mice.
High response rates to immunosuppressive therapy (IST) have suggested that acquired aplastic anemia (AA) is an immune-mediated process7ŌĆō9). Activated cytotoxic T lymphocytes and their soluble products are possible final effecters for hematopoietic injury10)
Based on the importance of activation-induced cell death (AICD) in autoimmune disease and autoimmune phenotype of AA, we speculated that the abnormal AICD of T lymphocytes in AA might inhibit elimination of activated T lymphocytes and thereby cause the AA phenotype. We investigated both T lymphocytes death and Fas antigen expression by activation in AA, and also compared the results of patients with recovered AA.
MATERIALS AND METHODS
1. Patients
This study included 20 patients with aplastic anemia (13 newly diagnosed, 7 recovered AA after IST) who were admitted between June 1995 and March 1996, and 6 normal controls. We studied the expression of Fas antigen on fresh T lymphocytes of all patients, and investigated the AICD and Fas expression by activation in 5 newly-diagnosed AA, 5 normal controls and 5 AA in complete response (CR).
2. Preparation of T lymphocytes
Peripheral blood samples were obtained from patients with newly-diagnosed severe AA, patients with AA in CR after IST and normal controls. Peripheral blood mononuclear cells were isolated from heparinized peripheral blood by Ficoll-Hypaque density gradient centrifugation. Then CD2+ cells were prepared by immunomagnetic bead methods, as described in detail elsewhere11). The subsets prepared in this way were regularly 90ŌĆō98% positive for CD2 or CD3 phenotype.
3. T lymphocytes activation
T lymphocytes were cultured with a RPMI-1640 medium supplemented with L-glutamine, 50 U/ml penicillin G, 50 ╬╝g/ml streptomycin and 10% of FBS at a concentration of 1.5├Ś106 cells/ml at 37┬░C, 5% CO2 for the times indicated. Interleukin-2 (IL-2) (Eurocetus, UK) and PHA (Sigma, USA) were present at a concentration of 200 U/ml and 50 ╬╝g/ml, respectively. Media, IL-2 and PHA were replenished every 3 days and cell numbers were readjusted.
4. Flow cytometric analysis
Fas antigen expression of T lymphocytes by activation was investigated using fluorescein isothiocyanate conjugated anti-Fas monoclonal antibody (IgG; UBI, NY, USA).
5. Quantitation of AICD
We used the cell death detection ELISA kit (Boehringer Manheim, Germany) for the quantitation of cell death. This assay is based on the quantitative sandwich-enzyme-immunoassay principle using mouse monoclonal antibodies directed against DNA and histones, respectively. This allows for the specific determination of mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates. Briefly, at the time intervals indicated, 1├Ś104 cells were removed from culture and pelleted by centrifugation. The cell pellets were resuspended with 500 ╬╝l incubation buffer and incubated for 30 min at 4┬░C. After centrifugation, 400 ╬╝l supernants were obtained and the resulting supernants were prediluted 1:3 with incubation buffer. These sample solutions were added to anti-histone antibody (cion H 11-4; Boehringer Manheim) coated microtiter plates (MTP). After incubation for 90 min at room temperature, 100 ╬╝l of anti-DNA peroxidase were added to each MTP. Then, 100 ╬╝l of ABTS solution (Boehringer Manheim) were added and absorbance was measured at 405 nm.
6. Analysis of DNA fragmentation
To assess DNA fragmentation following activation, genomic DNA was isolated from 3-day activated T lymphocytes. Briefly, at 3 days of activation, 2├Ś106 cells were washed in PBS and pelleted in Eppendorf tubes, lysed in 0.5 ml of lysis buffer (10 mM EDTA; 50 mM Tris-HCI, pH 8; 1% SDS; and 250 ╬╝g/ml of proteinase K) and incubated for 60 min at 50┬░C. Nucleic acids were extracted from the digested lysates by phenol/chloroform extraction method and then precipitated overnight with cold 100% ethanol at ŌłÆ20┬░C. Nucleic acid precipitates were centrifuged for 15 min at 2000├Śg, vacuum dried and resuspended in ŌłÆ20┬░C ╬╝l of TE buffer and incubated with 250 ╬╝g/ml of RNase at 65┬░C for 5 min to remove RNA. Electrophoresis of the resulting DNA samples was carried out at 5 V/cm in 2% agarose gels made in TBE buffer containing 0.5 ╬╝g/ml of ethidium bromide. Fractionated DNA was visualized by exposure to UV light and photographed.
7. Anti-Fas Ab sensitivity of activated T lymphocytes
At 3 days of activation, T lymphocytes were removed from culture, pelleted by centrifugation and resuspended at a concentration of 1├Ś104 cells/ml in original media. Anti-human Fas Ab (IgM; UBI, NY, USA) was added at a final concentration of 500 ng/ml and the cells were cultured for the times indicated at 37┬░C. After incubation, cell death was quantitated as described earlier.
RESULTS
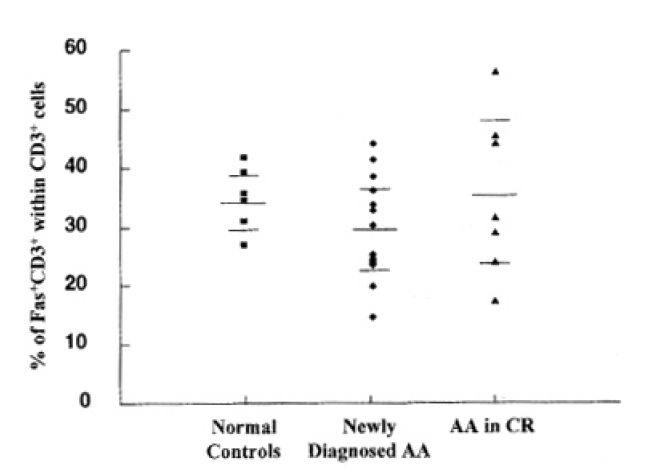
1. Expression of Fas antigen on fresh lymphocytes
Before the investigation of cell death and Fas antigen expression by activation, we analyzed the baseline expression of Fas antigen on T lymphocytes. There was no significant difference of Fas antigen expression on freshly-isolated T lymphocytes among newly-diagnosed severe AA, normal controls and patients with AA in CR after IST (Fig. 1).
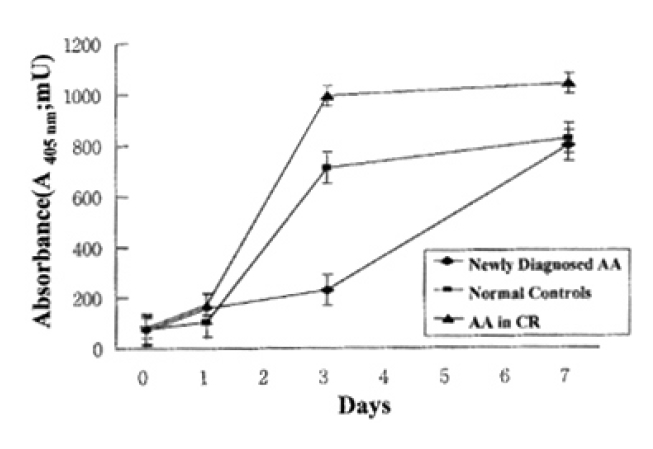
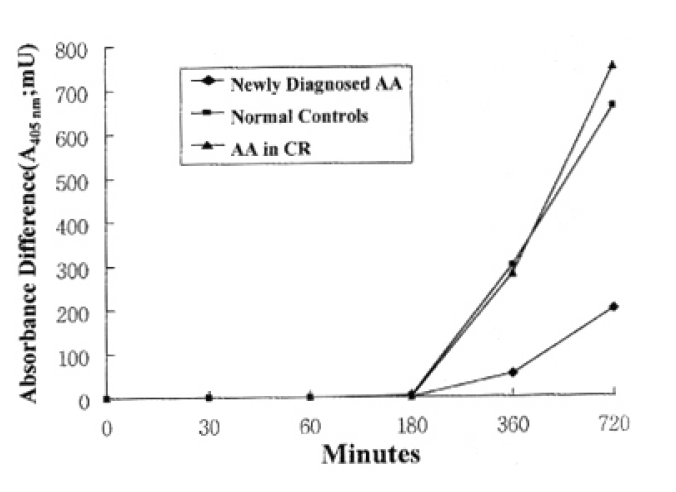
2. Quantitation of T lymphocytes death by activation
In normal controls, death of T lymphocytes was greatly increased at 3 days of activation. Patients with AA in CR showed a similar cell-death pattern, i.e., significant enhancement of cell death at 3 days of activation. In contrast, newly-diagnosed AA patients did not show the enhancement of cell death at 3 days of activation, but enhancement of cell death was noted at 7 days of activation (Fig. 2)
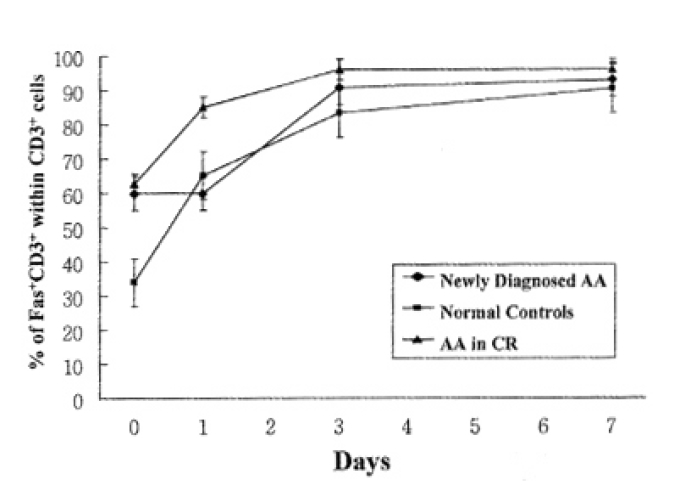
3. Expression of Fas antigen on activated T lymphocytes
In addition to quantitation of T lymphocytes death by activation, we examined the Fas antigen expression by T lymphocytes at the same time intervals to correlate ceil-death pattern with Fas antigen expression by activation. In normal controls and patients with AA in CR, Fas antigen expression on T lymphocytes was increased above baseline at day 1 of activation. But newly-diagnosed AA patients did not show any increase of Fas antigen expression of T lymphocytes at day 1 of activation, but enhanced Fas antigen expression at day 3 of activation (Fig. 3).
4. Anti-Fas antibody sensitivity of activated T lymphocytes
To investigate the difference of Fas-mediated death of activated T lymphocytes between each group, we treated the anti-fas antibody to T lymphocytes at 3 days of activation, and analyzed the time-course kinetics of induction of cell death by anti-Fas antibody. In normal controls and patients with AA in CR, activated T lymphocytes acquired significantly increased sensitivity to the anti-Fas antibody at 6 hours of treatment, while a reduced degree of sensitivity increment to anti-Fas antibody was noted in newly-diagnosed AA patients (Fig. 4).
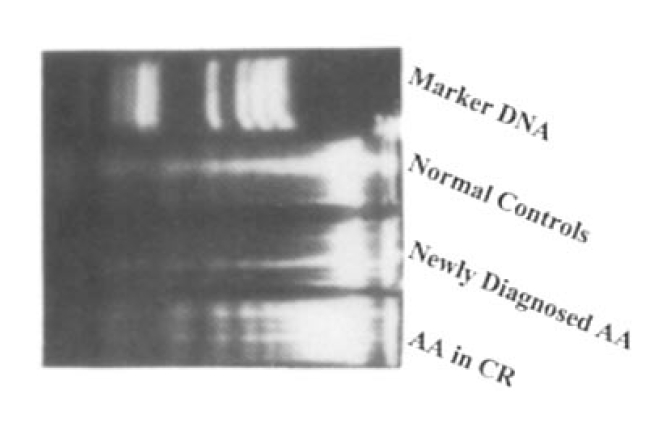
5. Documentation of DNA fragmentation
In all groups, activated T lymhpocytes showed characteristic DNA fragmentation at day 3 of activation(Fig. 5.)
DISCUSSION
Clinical and laboratory evidence suggests that hematopoietic failure in AA is likely to be mediated by cytotoxic T lymphocytes that are detectable in blood and marrow. These cells produce the inhibitory cytokine r-interferon and tumor necrosis factor-╬▓, both of which induce cell death in the CD34+ components12). Although findings of lymphocyte activation have been broadly confirmed, the biologic characteristics of self-reactive, cytotoxic T lymphocytes are not precisely defined.
Under various experimental conditions, activation-induced cell death can be triggered in vivo and in vitro in mature peripheral T lymphocytes1,13) Several studies have previously reported that monoclonal antibodies directed the CD3/TCR complex and mitogenic lecitins, such as PHA, activate resting T lymphocytes but induce cell death. These observations lend support to the notion that apoptosis may play an important role in the regulation of cellular immune response. In addition, the elimination of antigen-reactive T lymphocytes by apoptosis may be one of several mechanisms that contribute to the establishment of tolerance in the peripheral immune system.
Apoptosis is believed to play an important role in the deletion of autoreactive or unwanted T lymphocytes in two different phases during the ontogeny of the immune response. First, the encounter of self-antigens in the thymus leads to T lymphocytes deletion characterized by apoptotic cell death. Thymic T lymphocytes death, however, appears to be independent of Fas antigen, because mice homozygous for the Ipr mutation, which results in expression of a defective Fas molecule6,14), appears to delete autoreactive T lymphocytes in a normal manner15). Second, chronically stimulated mature T lymphocytes can be eliminated in the periphery by the process of AICD1). Several groups showed that peripheral clonal deletion and the elimination of activated T lymphocytes are impaired in Ipr mice16,17), and recent analyses of AICD of T lymphocytes demonstrated that antagonists of Fas are able to block AICD induced by CD3 antibody, or by phorbol ester plus calcium ionophore, or by staphylococcal enterotoxin B (SEB) supernant (Alderson et al. 1995). These results show that Fas system is critically involved in AICD of mature T lymphocytes, and it seems likely that Fas system is the prime mediator of the peripheral deletion of T lymphocytes and maintenance of peripheral self-tolerance.
We have used the sensitive ELISA method for determination of cytoplasmic histone-associated DNA fragments to assess cell death of human T lymphocytes by activation with IL-2 and PHA, and have confirmed the presence of cell death in these cells by gel analysis. Our data confirm the work of others that normal lymphocytes gradually undergo apoptosis when activated18,19). The major finding of this study is that T lymphocytes derived from patients with AA undergo more delayed AICD than T lymphocytes from normal controls. In addition to delayed AICD, T lymphocytes from newly-diagnosed AA patients also exhibited a delayed increase of Fas antigen expression by activation, and decreased sensitivity to anti-Fas antibody than normal controls. These results suggest that abnormal Fas system might be responsible for delayed AICD and, eventually, autoreactivity of T lymphocytes in AA.
IST reduces the activated lymphocyte numbers in recovered patients 20,21). In some series, the inhibitory activity of T lymphocytes has disappeared after immunosuppressive therapy22,23). But the specific target subsets of T lymphocytes to IST is unknown. Considering our results that delayed AICD and decreased Fas antigen expression by activation of T lymphocytes from AA were recovered to normal range after successful IST, IST may eliminate this abnormal T cell clone showing delayed AICD.
The findings of typical DNA fragmentation of AICD in newly-diagnosed AA indicate that delayed AICD in AA is more associated with abnormal regulation of Fas antigen expression than Fas-signaling defects. Recently, it has been determined that there is an inverse expression between bcl-2 and Fas antigen in activated lymphocytes. Further studies will be required to verify the role of bcl-2 and other molecules on the expression of Fas antigen in AA.
Although it remains important to address the underlying defect of delayed AICD of T lymphocytes in AA, our data suggest that the delayed AICD associated with abnormal Fas system might be responsible for immune pathophysiology of AA. Until further information on in vivo apoptosis in AA patients is revealed, it will remain unclear whether delayed AICD is observed in vivo. Also it is not apparent that abnormal AICD is pathogenic for all AA patients. Further investigation of apoptosis in AA may yield important new insights into the fundamental pathogenesis of this disease.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





