 |
 |
| Korean J Intern Med > Volume 23(1); 2008 > Article |
|
Abstract
Background/Aims
We used flexible starting doses and early titration of atorvastatin to determine the rate of achievement of a low-density lipoprotein cholesterol (LDL-C) target for hyperlipidemic patients with type 2 diabetes mellitus.
Methods
This study was a multicenter, open-label, prospective trial of atorvastatin therapy in hyperlipidemic patients with type 2 diabetes. The patients were divided into three groups according to initial LDL-C levels (130-149, 150-159 andŌēź160 mg/dL), and 10, 20, and 40 mg of atorvastatin was administered to each group, respectively. If LDL-C did not reach the 100 mg/dL target level at four weeks after initiation of treatment, the doses were increased by one step. Endothelial function tests and plasminogen activator inhibitor (PAI)-1 levels were measured in 41 patients.
Results
Groups of 62, 18, and 69 patients were started on 10, 20, and 40 mg of atorvastatin, respectively, and 91% of the patients achieved the LDL-C target after four weeks of treatment. The overall percentage of patients achieving the LDL-C target after eight weeks of treatment was 89.3%: 87.5% in the 10 mg group, 86.4% in the 20 mg group, 93.9% in the 40 mg group, and 66.7% in the 80 mg group. A statistically significant reduction was observed in the mean percentage change in flow-mediated endothelium-dependent dilatation after eight weeks of treatment (p<0.0001).
The development of type 2 diabetes mellitus (T2DM) is accompanied by multiple risk factors for developing coronary heart disease (CHD), such as hypertension, obesity, and dyslipidemia. Among modifiable risk factors, the association between plasma low-density lipoprotein cholesterol (LDL-C) and CHD risk is well established, and this association holds true for individuals with diabetes even at low plasma LDL-C concentrations 1-3). Based on several large-scale randomized controlled trials4-6), the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), stated that diabetes is a coronary heart disease equivalent7). Thereafter, the American Diabetes Association (ADA) recommended more aggressive pharmacological therapy in the management of dyslipidemia for adults with diabetes. The LDL goal was set at <100 mg/dL. The ADA recommended that drug therapy should be initiated at the same time as lifestyle intervention is started if the LDL-C level is 130 mg/dL or greater in the absence of coronary, peripheral, and cerebral vascular diseases, whereas it should be initiated at an LDL-C level of 100 mg/dL or greater in their presence8). However, among patients treated with statins, the rate of achieving a reduced LDL-C goal after 16 weeks of treatment was 65% with the usual dose of atorvastatin, but only 19% with pravastatin9, 10). This suggests that a more practical and aggressive strategy for rapidly and effectively reaching a given treatment goal of reduced LDL-C levels is urgently needed for patients with diabetes.
So far, there has been no 'gold standard' for the starting dose and dose titration of a statin according to a patient's LDL-C levels, toward the end of achieving the target level. If patients were started on a dose of a statin by anticipating its effect instead of relying on dose escalation, then the reduced LDL-C target might be reached more rapidly and effectively. Furthermore, appropriate dose adjustment of the statin based on LDL-C levels may also be essential for achieving the goal. The cholesterol-lowering effect of atorvastatin usually reaches its maximum level at four weeks, with subsequent maintenance of a similar effect throughout the period of therapy11). However, there are no clearly established guidelines for the early aggressive titration of statin doses.
Besides the traditional risk factors for coronary heart disease, nontraditional risk factors such as insulin resistance, endothelial dysfunction, impaired fibrinolysis evidenced by elevated levels of plasminogen activator inhibitor type 1 (PAI-1), low-grade inflammation (hs-CRP elevation), microalbuminuria, and vascular wall abnormalities are considered to be contributors to the excess risk of CHD associated with diabetes, which cannot be fully explained in the context of the traditional risk factors alone12). Statins also provide beneficial effects for the nontraditional risk factors of CHD. These benefits include restoration of endothelial function, stabilization of atherosclerotic plaques, and decreases in oxidative stress and vascular inflammation. In addition to its lipidlowering effect, atorvastatin has also shown an anti-inflammatory effect associated with improved endothelial-dependent dilation in a prospective study13, 14).
In this study, we first tested the effects of initial flexible doses and early aggressive dose titration of atorvastatin according to the patient's LDL-C levels as a practical guideline for statin therapy. Second, we measured the non-lipid-lowering effects of atorvastatin in the same patients, based on flow-mediated endothelium-dependent dilation (FMD), flow-mediated endotheliumindependent dilation (EID), and PAI-1 levels.
Men and women aged 18-80 years were eligible for the study if they were hyperlipidemic T2DM patients with LDL-C levelsŌēź130 mg/dL or glycated hemoglobin (HbA1c) values of Ōēż10% and triglyceride levels of Ōēż400 mg/dL at baseline. Patients were not included in this study if they had type 1 diabetes mellitus or serious medical illness, or if they had been treated with fibrates, corticosteroids, azole-derivative antifungal agents, or niacin. Patients were also excluded if they were women of childbearing potential not using contraception, or if they had secondary causes of hyperlipoproteinemia (thyroid-stimulating hormone Ōēź1.5 times the upper limit of normal, blood urea nitrogenŌēź30 mg/dL, or creatinineŌēź2.0 mg/dL at baseline), uncontrolled hypertension (sitting systolic blood pressure [BP]>160 mmHg, sitting diastolic BP>100 mmHg), hepatic disease, or impaired hepatic function (aspartate or alanine aminotransferase [AST/ALT] levels >two times the upper limits of normal at baseline). Other exclusion criteria were: creatine phosphokinase (CPK)>three times the upper limit of the normal range at baseline, body mass index (BMI)>32 kg/m2, a history of intolerance or hypersensitivity to HMG-CoA reductase inhibitors, or abuse of alcohol or other recreational drugs. Patients with cardiac arrhythmia were not included in the group receiving endothelial function measurements.
This multicenter, open-labeled, prospective study was conducted at 11 university-affiliated diabetes centers in Korea. Patients received appropriate doses of atorvastatin according to designed criteria for a total of eight weeks. Patients who were on any other lipid-lowering medications before enrollment underwent a four-week washout period before being placed on the standard protocol. The patients were divided into three groups according to their initial LDL-C levels (130-149 mg/dL, 150-159 mg/dL, and Ōēź160 mg/dL), and 10, 20, and 40 mg of atorvastatin was administered as an initial dose in each group, respectively.
If the patient's LDL-C levels did not reduce to the target level after four weeks of treatment, the dose was increased by one step, up to 80 mg. If the LDL-C levels met the target after four weeks of treatment, the dose was maintained at the same level. All the patients were encouraged to adhere to their allocated diet or exercise therapy during the entire study period. The study was conducted according to the principles of the Declaration of Helsinki; it was approved by institutional review boards at all centers, and written informed consent was obtained from all patients.
The sample size was determined to be adequate to address the study hypothesis that atorvastatin treatment would enable patients to achieve their designated LDL-C target quickly, with either no titration or just one titration step, provided the starting dose was appropriate for the level of LDL-C reduction required by the patient's cardiovascular risk category. 151 patients were needed to make the study adhere to our assumed percentages: 50% of patients reaching goal, 20% dropouts, and a lower bound of a one-sided 95% confidence interval with a width of 15% of the target population. However, we determined the final sample size of 198 patients to secure adequate patients.
Fasting serum lipid profiles in all centers, and endothelial function and PAI-1 level measurements in three centers, were assessed for efficacy. Patients were instructed to fast for 12 h before each test, and they had no vigorous exercise or change in diet the day before the test. Total cholesterol (TC) and triglyceride (TG) levels were measured enzymatically after precipitation of the other lipoproteins. The LDL-C level was calculated using Friedewald's equation if the serum triglyceride level was Ōēż400 mg/dL. The blood glucose level was measured using an automatic enzymatic method, and the glycated hemoglobin (HbA1c) level was determined by high-performance liquid chromatography (HPLC; Variant II HbA1c analyzer; Bio-Rad, Montreal, Quebec, Canada) with a reference range of 4.4~6.4% of total hemoglobin.
Endothelial function was assessed at baseline and at week eight by echocardiography with monitoring of electrocardiograms, which measured brachial artery flow-mediated endotheliumdependent dilation (FMD) and brachial artery flow-mediated endothelium-independent dilation (EID). Echocardiography was performed in three of the centers, and the measurements of FMD and EDI by recorded images were performed by one investigator. PAI-1 levels were measured using enzyme-linked immunosorbent (ELISA) kits (Biopool, Umea, Sweden) at baseline and at week eight. Physical examinations, measurements of blood pressure and body weight, and laboratory tests were performed at every visit, and any adverse event (AE) was recorded for safety evaluation.
The primary efficacy variable was the percentage of patients achieving their National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Adult Treatment Panel III (NCEP ATP III) target of LDL-C<100 mg/dL at eight weeks. The secondary efficacy variables included the percentage changes, from baseline to eight weeks, of LDL-C, HDL-C, triglycerides, total cholesterol, non-HDL cholesterol, (LDL cholesterol)/(HDL cholesterol) ratio, PAI-1, FMD, and EID.
All patients in the per protocol (PP) population were analyzed for efficacy. The PP population consisted of patients who were assigned to a starting dose of atorvastatin, who had both baseline and eight-week LDL cholesterol efficacy measurements, and who adhered to the protocol requirements. For safety analysis, patients who had at least one medication were included, and the last-observation-carried-forward (LOCF) approach was used to evaluate missing data.
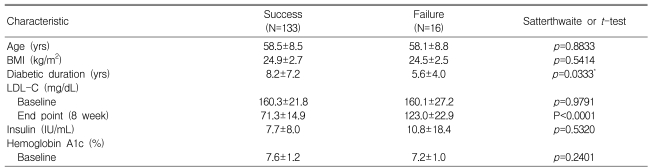
Descriptive statistics such as mean, standard deviation, frequency, and percentage have been used to present the distributions of baseline characteristics, efficacy, and safety measurements. The primary efficacy variable was the percentage of patients achieving their LDL-C NCEP ATP III target at eight weeks. The overall rate of achieving the target LDL-C 95% confidence intervals (CI) are presented. The Chi-square test was used to compare the difference of rates among starting doses of atorvastatin. The baseline characteristics of the two outcome groups (success or failure to achieve the target) were compared using Satterthwaite or Student's t-tests.
The secondary efficacy variables were the percentage changes from baseline to eight weeks in the following lipid parameters: LDL cholesterol, HDL cholesterol, TG, TC, non-HDL cholesterol, (LDL cholesterol)/(HDL cholesterol) ratio, FMD, and EID; and the percent change from baseline to eight weeks in the PAI-1 level. Mean percentage changes from baseline in lipid parameters and PAI-1 were compared using paired t-tests.
We used SAS version 8.01 for statistical analysis; all tests were two-sided with significance assumed at p<0.05.
Of 322 subjects screened, evaluable safety data were available for 209 patients. Among them, 149 patients completed this study, and all the analyzed data for the efficacy are based on this group (Figure 1). The mean age was 58.4┬▒8.5 y (range 32-76 y), and 106 (71.1%) of the patients were women (Table 1). According to the initial LDL-C levels, 62 (41.6%), 18 (12.1%), and 69 (46.3%) of the 149 patients were assigned to receive 10, 20, and 40 mg doses of atorvastatin, respectively.
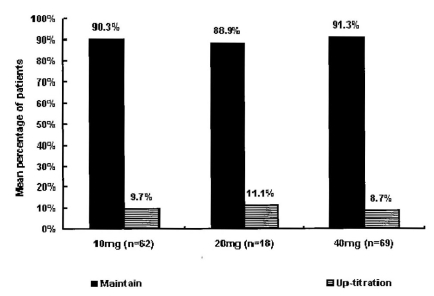
After the first assessment at week four, 9.7% of patients in the 10 mg group, 11.1% of patients in the 20 mg group, and 8.7% of patients in the 40 mg group were changed to higher doses. Thus, 56 (37.6%), 22 (14.8%), 65 (43.6%) and 6 (4%) of the 149 patients received 10, 20, 40, and 80 mg of atorvastatin, respectively, starting at this point (Figure 2, 3).
At the end of the study in all dose groups, 89% of all patients achieved the target LDL-C level (95% CI, 84~94%). The target was achieved by 66.7% of patients in the 80 mg group, 93.9% in the 40 mg group, 86.4% in the 20 mg group, and 87.5% in the 10 mg group (Table 2).
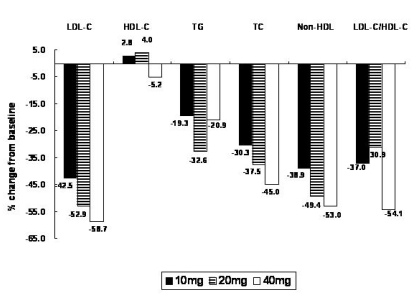
Serum parameters from baseline to eight weeks were significantly lowered for LDL-C (-51.2┬▒15.6%), TC (-38.0┬▒11.9%), TG (-21.6┬▒42.0%), and non-HDL-C (-47.1┬▒13.6%) (Figure 4). Among the lipid parameters, LDL-C, TC, and non-HDL-C values were significantly decreased after eight weeks compared with baseline levels, in a dose-dependent manner (p<0.0001). Atorvastatin was more effective in lowering TG in the 20 mg group and inducing greater change in LDL-C/HDL-C ratio in the 40 mg group than the other groups, but there were no statistically significant differences among groups, respectively (p=0.2128, p=0.9875). Interestingly, the HDL-C level of the 40 mg group was significantly lowered compared with the 10 mg and 20 mg groups (p=0.0079).
The rates of patients achieving their target did not show any significant differences in relation to covariates (Table 3). Of the 149 total patients, 135 (90.6%) reached the target level of LDL-C at four weeks, but 12 (8%) of these patients had elevated LDL-C levels (>100 mg/dL) at eight weeks. Fourteen patients needed to increase their dosage at four weeks because they did not reach the target at four weeks ('failure'). The success and failure groups did not differ significantly in age, BMI, baseline LDL-C insulin, or HbA1c levels, but did show differences in the duration of diabetes (success vs. failure, 8.2┬▒7.2 vs. 5.6┬▒4.0 years, p<0.05) (Table 3).
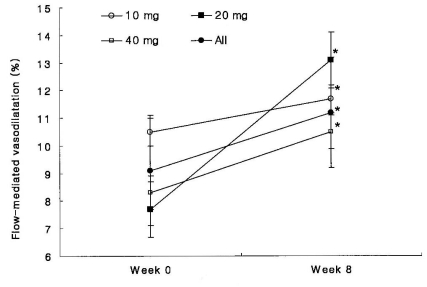
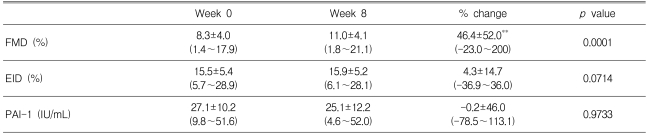
The baseline clinical characteristics of the endothelial function study subpopulation are listed in Table 4. Atorvastatin supplementation improved FMD after eight weeks of treatment (Figure 5), but EID was not significantly affected. PAI-1 tended to decrease in patients receiving atorvastatin, but this parameter change did not reach statistical significance.
Among the 209 original study subjects, 30 patients treated with atorvastatin reported 41 adverse events. Of these, 24 patients (12%) reported adverse events that were considered by the investigators to be possibly, probably, or definitely associated with treatment. Most of these events were mild to moderate in intensity. The rate of adverse events increased with increasing drug dosage (10%, 14%, and 18% in the 10, 20, and 40mg groups, respectively. p=0.3783). The most commonly reported events were abdominal pain, dizziness, headache, and dyspepsia. Ten patients (4.7%) withdrew because of these adverse events. Two patients discontinued the drug because of elevation of their ALT levels (from 18 to 208 IU/L and from 18 to 178 IU/L after four weeks of treatment) and one of them because of an elevation of CPK level (from 97 to 346 IU/L). There were no cases of persistent elevations in serum transaminases (greater than three times the upper limit of normal) or reports of myopathy in any treatment group. No serious adverse event was reported (Table 5).
In this study, we aimed to develop an intensive treatment guideline for lipid-lowering therapy that would achieve an LDL-C target in T2DM patients rapidly and effectively. We therefore observed the rate of achieving the NCEP treatment goal within eight weeks of atorvastatin therapy, with the starting dose stratified according to the individual patient's baseline LDL-C level. Atorvastatin, administered at 10, 20, 40, and 80 mg, produced effective reductions in total cholesterol and LDL-C within eight weeks.
Many primary and secondary prevention studies provide strong support for the use of moderate doses of statins in patients with diabetes. Although the NCEP and ADA guideline for the LDL-C target is 100 mg/dL, only a few studies support the idea that lower LDL-C levels lead to better cardiovascular outcome. The Heart Protection Study (HPS), which had the largest diabetes subgroup, demonstrated a consistent (close to 25%) reduction and a 5~7% absolute risk reduction in cardiovascular events with statin therapy regardless of initial LDL-C levelseven among patients whose initial levels were below the NCEP target of 100 mg/dL15). For better cardiovascular outcome, aggressive therapy with statins is warranted in diabetic subjects. However, the effectiveness of the available statin medications in lowering LDL-C is variable. Most statins are capable of achieving the LDL-C goal in subjects with LDL-C levels of less than 170 mg/dL. Atorvastatin at 40 mg or simvastatin at 80 mg could achieve the goal in subjects with LDL-C levels of 170-189 mg/dL, but only atorvastatin at 80 mg and the recently developed drug rosuvastatin could do so for subjects with LDL-C levelsŌēź190 mg/dL16). To achieve the desired target of 100 mg/dL, dose adjustment would inevitably be needed in subjects with higher LDL-C levels. Considering the time and cost efficacy, starting-dose stratification seemed to be a valuable method in daily clinical practice and was eventually revealed to be so in this study, as shown by the LDL-C target achievement rate of 89% at eight weeks. Furthermore, most of the LDL-C reduction was achieved within four weeks. The clinical characteristics of subjects who achieved the target level were not different from those of who did not achieve it, except for the overall duration of diabetes mellitus. However, because most patients achieved the target level, the 'failure' group was small. If more subjects had been studied, differences between the success and failure groups might have been more clearly defined.
The mean percentages of LDL-C, total cholesterol, and non-HDL cholesterol were all reduced in a dose-dependent manner from baseline. The mean LDL-C changes were higher than the CURVES study achieved with initial dose randomization17). Initial dose stratification could explain the differences in the effectiveness of the same dosage of the same drug. Moreover, the HDL cholesterol level was slightly increased in the 10 mg and 20 mg groups, and decreased in the 40 mg group, after eight weeks of treatment. The degree of TG decrease was also smaller in the 40 mg group than in the 20 mg group, although this was not statistically significant.
Endothelial dysfunction occurs early in the atherosclerotic process and is associated with other cardiovascular risk factors18); impairment of fibrinolytic activity has been reported in dysmetabolic conditions associated with atherosclerosis, such as diabetes mellitus, and is related to endothelial function19). Recent studies have suggested that statins might have pleiotropic effects on endothelial function beyond their lipid-lowering effect. Several mechanisms by which statins might improve endothelial function, including increasing the synthesis of nitric oxide, decreasing the synthesis of endothelin-1, and inhibiting LDLcholesterol oxidation, have been suggested20-22). We showed that treatment with atorvastatin for eight weeks significantly improved endothelial function in patients with type 2 diabetes. Atorvastatin also reduced the PAI-1 levels, but the effect failed to reach statistical significance, probably because the study was short and patients showed considerable variation in their PAI-1 levels. Although further studies are needed to elucidate the mechanisms, these results suggest that early treatment with atorvastatin could reduce the incidence of coronary heart disease in patients with diabetes.
A few adverse events were reported in this study. Ten patients withdrew because of drug-related events. Among them, three showed drug-related adverse biochemical changes (elevations in ALT or CPK levels), which were reversible after drug discontinuation. Thus, atorvastatin appears to be relatively safe for the treatment of patients with T2DM, even at high doses.
In conclusion, an initial flexible dose of atorvastatin treatment based on initial LDL-C levels can achieve a target LDL-C level effectively, rapidly, and safely in most hyperlipidemic patients with T2DM. Therefore, this dose-escalation schedule might be helpful, especially for high-risk patients seen in daily clinical practice. However, the clinical significance remains to be clarified by future studies.
References
1. Eisenberg DA. Cholesterol lowering in the management of coronary artery disease: the clinical implications of recent trials. Am J Med 1998. 104:2SŌĆō5SPMID : 9550499.

2. Kannel WB. Range of serum cholesterol values in the population developing coronary artery disease. Am J Cardiol 1995. 76:69CŌĆō77C.

3. Goldberg RB, Mellies MJ, Sacks FM, Moye LA, Howard BV, Howard WJ, Davis BR, Cole TG, Pfeffer MA, Braunwald E. Cardiovascular events and their reduction with pravastatin in diabetic and glucose intolerant myocardial infarction survivors with average cholesterol levels. Circulation 1998. 98:2513ŌĆō2519PMID : 9843456.


4. Collins R, Armitage J, Parish S, Seligh P, Peto R. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 5963 people with diabetes: a randomized placebo controlled trial. Lancet 2003. 361:2005ŌĆō2016PMID : 12814710.


5. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003. 348:383ŌĆō393PMID : 12556541.


6. Wiklund O, Haversen L, Pettersson C, Hulten LM. How can we prevent cardiovascular disease in diabetes. J Intern Med 2007. 262:199ŌĆō207PMID : 17645587.


7. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Cholesterol in Adults (Adult treatment panel III). JAMA 2001. 285:2486ŌĆō2497PMID : 11368702.

8. Haffner SM. Dyslipidemia management in adults with dabetes. Diabetes Care 2004. 27(Suppl 1):S68ŌĆōS71PMID : 14693930.


9. Bertolini S, Bon GB, Campbell LM, Farnier M, Langan J, Mahla G, Pauciullo P, Sirtori C, Egros F, Fayyad R, Nawrocki JW. Efficacy and safety of atorvastatin compared to pravastatin in patients with hypercholesterolemia. Atherosclerosis 1997. 130:191ŌĆō197PMID : 9126664.


10. Insull W, Kafonek S, Goldner D, Zieve F. Comparison of efficacy and safety of atorvastatin (10mg) with simvastatin (10mg) at six weeks. Am J Cardiol 2001. 87:554ŌĆō549PMID : 11230838.


11. Heinonen TM, Stein E, Weiss SR, McKenney JM, Davidson M, Shurzinske L, Black DM. The lipid-lowering effects of atorvastatin, a new HMG-CoA reductase inhibitor: results of a randomized, double-masked study. Clin Ther 1996. 18:853ŌĆō863PMID : 8930429.


12. Fonseca V, Desouza C, Asnani S, Jialal I. Nontraditional risk factors for cardiovascular disease in diabetes. Endocr Rev 2004. 25:153ŌĆō175PMID : 14769830.


13. Marchesi S, Lupattelli G, Siepi D, Schillaci G, Vaudo G, Roscini AR, Sinzinger H, Mannarino E. Short-term atorvastatin treatment improves endothelial function in hypercholesterolemic women. J Cardiovasc Pharmacol 2000. 36:617ŌĆō621PMID : 11065222.


14. Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes. Diabetes Metab 2001. 27:436ŌĆō447PMID : 11547217.

15. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002. 360:7ŌĆō22PMID : 12114036.


16. Jones PH, Hunninghake DB, Ferdinand KC, Stein EA, Gold A, Caplan RJ, Blasetto JW. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non high density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia. Clin Ther 2004. 26:1388ŌĆō1399PMID : 15531001.


17. Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol 1998. 81:582ŌĆō587PMID : 9514454.


18. Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 1986. 315:1046ŌĆō1051PMID : 3093861.


19. Gough SC, Grant PJ. The fibrinolytic system in diabetes mellitus. Diabet Med 1991. 8:898ŌĆō905PMID : 1838039.


20. Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res 2000. 47:648ŌĆō657PMID : 10974215.


21. Hernandez Perera O, Perez Sala D, Navarro Antolin J, Sanchez Pascuala R, Hernandez G, Diaz C, Lamas S. Effects of the 3-hydroxy-3-methylglutaryl CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest 1998. 101:2711ŌĆō2719PMID : 9637705.



APPENDICES
Appendix
Participants of the Atorvastatin Study Group in Korea are as follows: H.Y. Son, K.H. Yoon, Y.H. Choi, H.S. Kwon, S.H. Ko, Department of Internal Medicine; The Catholic University of Korea; I.K. Lee, H.S. Kim, K.K. Park, M.J. Kim, Department of Internal Medicine, The Keimyung University, School of Medicine; J.T. Woo, Y.S. Kim, Department of Internal Medicine, Kyung Hee University College of Medicine; D.S. Choi, Department of Internal Medicine, Korea University College of Medicine; M. K. Lee, H.J. Kim, Department of Internal Medicine, SungKyunKwan University School of Medicine; K. S. Park, H. K. Lee, Seoul National University College of Medicine; J. Y. Park, E. H. Koh, University of Ulsan College of Medicine; H. C. Lee, B. S. Cha, Yonsei University College of Medicine; M. Y. Chung, D. H. Cho, Chonnam National University College of Medicine; Y. K. Kim, I. J. Kim, Pusan National University College of Medicine; Y. K. Kim, B. J. Ku, Chungnam National University College of Medicine.
Figure┬Ā1
Patient population and study protocol. Among the 322 patients screened, 149 completed the protocol.
Figure┬Ā3
Dosage titration according to 1st LDL-C target at week 4 (n=149). After 4 weeks, 9.7% of patients in the 10 mg group, 11.1% of patients in the 20 mg group, and 8.7% of patients in the 40 mg group were changed to higher doses.
Figure┬Ā4
Mean percent change of lipid parameters from baseline after 8 weeks (n=149). Black bar, 10 mg; shaded bar, 20 mg; white bar, 40 mg.
Figure┬Ā5
Flow-mediated endothelium-dependent dilation over time.
Data are means┬▒SD. *p<0.05 vs. before treatment.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



