 |
 |
| Korean J Intern Med > Volume 35(1); 2020 > Article |
|
Abstract
Myeloproliferative neoplasms (MPNs) are a heterogeneous group of clonal disorders characterized by the overproduction of mature blood cells that have an increased risk of thrombosis and progression to acute myeloid leukemia. Next-generation sequencing studies have provided key insights regarding the molecular mechanisms of MPNs. MPN driver mutations in genes associated with the JAK-STAT pathway include JAK2 V617F, JAK2 exon 12 mutations and mutations in MPL, CALR, and CSF3R. Cooperating driver genes are also frequently detected and also mutated in other myeloid neoplasms; these driver genes are involved in epigenetic methylation, messenger RNA splicing, transcription regulation, and signal transduction. In addition, other genetic factors such as germline predisposition, order of mutation acquisition, and variant allele frequency also influence disease initiation and progression. This review summarizes the current understanding of the genetic basis of MPN, and demonstrates how molecular pathophysiology can improve both our understanding of MPN heterogeneity and clinical practice.
Myeloproliferative neoplasms (MPNs) are clonal hematopoietic disorders characterized by the proliferation of differentiated hematopoietic cells [1]. The latest World Health Organization classification of MPN, released in 2017, includes chronic myeloid leukemia (CML), chronic neutrophilic leukemia (CNL), polycythemia vera (PV), primary myelofibrosis (PMF), essential thrombocythemia (ET), chronic eosinophilic leukemia and other, unclassifiable MPNs [2]. MPNs are considered rare diseases, but their incidence rates are increasing [3,4]. According to a recent nationwide population study in Korea, among all MPNs the incidence was highest for ET (2.4 cases per 100,000 population per annum; range, 2.0 to 3.0), followed by PV (1.2 cases per 100,000 population per annum; range, 1.0 to 1.5) and PMF (0.4 cases per 100,000 population per annum; range, 0.3 to 0.5) [5].
MPNs were characterized in 1951 by Dameshek [6] as an overproduction of mature blood cells including erythroid, granulocytic, and megakaryocytic lineages in response to an unknown myelostimulatory factor. He also described the overlap of the clinical and laboratory features of bone marrow hypercellularity, splenomegaly, myelofibrosis progression, and a blast phase [6].
Significant progress in genetic and cellular experimental approaches has allowed for rapid accumulation of molecular data regarding the basis of MPN. It is now known that mutations in tyrosine kinase and other related genes lead to constitutively active proteins, which serve as key myelostimulatory factors [7,8]. These findings have stimulated the development of new targeted treatments, such as Janus kinase (JAK) inhibitors [9]. In the present review, we summarize recent insights regarding the three main mechanisms of MPN pathophysiology: (1) somatic MPN-driver mutations that stimulate activation of JAK-signal transducer and activator of transcription (STAT), (2) cooperating driver mutations in myeloid genes, and (3) other genetic factors involved in the heterogeneity of the MPN clinical phenotype.
The constitutive activation of the JAK-STAT pathway is a hallmark of MPNs [7,8]. Certain somatic driver mutations in hematopoietic stem cells (HSCs) can provide a selective advantage and lead to the formation of clones with JAK-STAT pathway gene mutations and myeloid cell proliferation. Documented drivers include the JAK2 V617F and exon 12 mutations [10-13], mutations in MPL [14], mutations in exon 9 of CALR [15,16], and mutations in the CSF3R (Fig. 1) [17,18]. Recently, the CSF3R mutation was identified as a driver gene in CNL [17]. In addition, negative regulators of the JAK-STAT signaling pathway, including lymphocyte specific adaptor protein (SH2B3, also known as LNK) [19,20], casitas B-lineage lymphoma proto-oncogene (CBL) [21,22], and suppressor of cytokine signaling (SOCS) proteins [23] are inactivated at a low frequency in MPNs, highlighting the importance of the JAK-STAT signaling pathway in MPN pathogenesis.
JAK2, a cytoplasmic tyrosine protein kinase, is required for hematopoietic cytokine receptor signal transduction. The erythropoietin receptor (EPOR), thrombopoietin receptor (TPOR, also known as MPL), and granulocyte colony-stimulating factor receptor (G-CSFR) regulate erythrocytosis, thrombocytosis, and neutrophilia, respectively [24]. Hematopoietic cytokine receptors lack intrinsic kinase activity and thus rely on the associated tyrosine kinase, JAK2 [24]. Normally, interaction of the hematopoietic cytokine receptor with its ligands, such as erythropoietin (EPO), thrombopoietin (TPO), or G-CSF, results in receptor dimerization followed by autophosphorylation and transphosphorylation of the receptor and JAK2 [7]. The activated JAK2-receptor complex then recruits and phosphorylates substrate molecules, including STAT proteins, which leads to target gene transcription in the nucleus (Fig. 2A) [7].
Two types of JAK2 mutations are associated with MPN (Fig. 1A). The first is a valine to phenylalanine substitution at amino acid position 617 (V617F) in exon 14 of JAK2; this mutation is found in 95% of PV cases and 50% to 60% of ET and PMF cases [10-13]. The second mutation type comprises mutations in exon 12 of JAK2, most of which are in-frame small insertions or deletions [25]. JAK2 exon 12 mutations are observed in 1% to 2% of PV patients, most of whom are JAK2 V617F-negative [25,26]. These mutations are found exclusively in PV and are not associated with ET or PMF [26]. The most frequent mutations are N542-E543del (30%), K539L (14%), E543-D544del (12%), and F537-K539delinsL (10%) [25].
JAK2 mutations promote constitutive activation of the JAK-STAT pathway (Fig. 2B) [10,12]. The V617F mutation, which occurs in the pseudokinase domain (Janus homology 2 [JH2]), leads to loss of the normal auto-inhibitory function of JH2 through changes in JH1 (kinase domain)-JH2 conformation and results in activation of JAK2 [27]. The activated JAK2 mutant recapitulates the physiological response to cytokine binding. Subsequent downstream activation of intracellular signaling occurs via STAT proteins, mitogen-activated protein kinase (MAPK), and phosphoinositidie-3-kinase (PI3K), which play important roles in excessive myeloid cell proliferation and differentiation [27]. Mutations in exon 12 are located upstream of the JH2 domain, within the linker region between the Src homology 2 (SH2) and JH2 domains. JAK2 exon 12 mutations lead to increased phosphorylation of JAK2 and STAT compared to the JAK2 V617F mutations, and result in cytokine-independent activation of JH1 [26]. Patients with the JAK2 exon 12 mutation exhibit more marked erythrocytosis at a younger age [26], suggesting that increased JAK2 promotes the polycythemic phenotype. These differences may reflect differences in the strength of aberrant signaling via the EPOR.
MPL is a cell surface receptor for TPO [28]. Megakaryopoiesis and platelet production are regulated by TPO, which binds to the cytokine receptor MPL to induce signaling through the JAK-STAT pathway [28]. Gain-offunction mutations in exon 10 of the MPL lead to cytokine-independent growth (Fig. 1B) [28]. MPL mutations at amino acid position 515 (W515) are present in 3% of ET cases and 5% of PMF cases, with the most frequent mutations being W515L and W515K [29]. Several other substitutions at the same position have been reported, namely W515R, W515A, and W515G [30]. A rare germline mutation, MPL S505N, was also described in a hereditary form of thrombocytosis [31].
W515, located in the transmembrane domain of MPL, normally prevents spontaneous activation of MPL [28]. Most substitutions of W515 lead to the activation of MPL in the absence of TPO and result in constitutive activation of JAK2 and STAT protein signaling and cytokine autonomous growth (Fig. 2D) [29].
In 2013, whole-exome sequencing of MPN samples revealed recurrent somatic mutations in exon 9 of CALR, i.e., the gene that encodes calreticulin [15,16], which is a multifunction calcium-binding chaperone protein [32]. Calreticulin is involved in the regulation of calcium homeostasis and the quality control of newly synthesized glycoproteins in the endoplasmic reticulum (ER) [32].
CALR mutations are the second most common mutation in patients with MPNs (after JAK2 V617F), and are found in 20% to 25% of ET and 25% to 30% of PMF cases [15,33-36]. CALR mutations are rarely reported in PV [15,16]. A knock-in mouse model demonstrated that mutant CALR drives an ET-like and myelofibrosis phenotype, in contrast to JAK2 mutations, which promote a PV-like phenotype [37]. CALR gene mutations shift the reading frame of calreticulin by 1 base pair (bp), resulting in a new C-terminal peptide and the loss of the original KDEL (lysine, aspartic acid, glutamic acid, and leucine) amino acid sequence motif, which plays an important role in retention of calreticulin in the ER [38]. Although calreticulin is not a JAK-STAT signaling molecule, mutant calreticulin interacts with MPL via the new C-terminal peptide to activate downstream signaling (Fig. 2C) [39-43]. Similar to wild-type calreticulin, mutant calreticulin binds to MPL in the ER, but the interaction is reinforced by the positive charges of the new C-terminus [38,43]. Bound by mutant calreticulin, MPL is exported to the cell surface [28]. TPO-independent activation of MPL leads to dimerization and activation of JAK2 and downstream STAT proteins [40]. In addition to binding between mutant calreticulin and MPL, more recent reports showed that three tyrosine residues within the intracellular domain of MPL (Y591, Y626, and Y631), in addition to lectin activity of calreticulin, are also necessary for activation of the JAK-STAT signaling pathway [44].
To date, more than 50 MPN CALR mutations have been found, 80% of which are type 1 or type 2 mutations (Fig. 1C) [34,35]. The type 1 mutation is a 52 bp deletion (c.1092_1143del, p.L367fs*46), whereas the type 2 mutation is a 5 bp insertion (c.1154_1155insTTGTC, p.K385fs*47). The distribution of these CALR mutation types varies depending on the MPN subtype: in PMF, the type 1 mutation is more prevalent than type 2 (70% vs. 13%), but in ET, type 1 and type 2 mutations are similarly distributed (51% vs. 39%) [45]. Recent evidence suggests a relationship between CALR mutation type and MPN clinical features [40,45,46]. Type 1-like mutants are associated with a myelofibrosis phenotype and significantly higher risk of myelofibrotic transformation in ET [46]. In contrast, type 2-like mutants are associated with an ET phenotype, low thrombosis risk (despite higher platelet counts), and indolent clinical course [46]. These mutations showed different structural changes in the exon 9 of calreticulin; the type 1 mutation lost most of the wild-type sequence and calcium-binding sites, whereas type 2 remained closer to the wild-type sequence, retaining about half of the negatively charged original amino acids [15]. These distinctive patterns indicate that the calreticulin mutation type may differentially impair the calcium binding activity of mutant calreticulin [46].
A mutation in CSF3R, which encodes G-CSFR, was recently identified as a CNL driver mutation [17,18]. This mutation activates the receptor, promoting proliferation of mature neutrophils, which are a hallmark of CNL [47]. Differential diagnosis of CNL and atypical CML (aCML), is challenging due to overlapping clinical features such as leukocytosis, splenomegaly, and bleeding diatheses [48]. Thus, the recent discovery of CSF3R mutations has facilitated the differential diagnosis of these disorders. The activating CSF3R T618I mutation is found in more than 80% of CNL patients, but is absent in aCML patients [18]. In 2017, the World Health Organization guidelines were updated to include CSF3R mutations as one of the diagnostic criteria for CNL [49].
The extracellular domain of CSF3R includes a membrane proximal region that is important for granulocytic proliferation, and a cytoplasmic region that regulates granulocytic differentiation and function [18,50]. The most common mutation in CNL, CSF3R T618I, is an activating mutation in the membrane proximal region that results in dimerization of the receptor independent of ligand binding and constitutive activation of the JAK-STAT pathway (Fig. 1D and 2E) [17].
Mutations in JAK2, CALR, and MPL are mutually exclusive and account for more than 90% of ET and PMF cases [33]. However, in 10% of ET and 5% to 10% of PMF cases, the disease-driving mutation is unknown, and these cases are referred to as triple-negative [33]. Typical diagnostic workup of MPN patients involves analysis of the JAK2 exon 14, MPL exon 10, and CALR exon 9 for the presence of canonical somatic mutations. However, approximately 10% of triple-negative ET and PMF patients have mutations outside of MPL exon 10 or JAK2 exon 14, and these noncanonical mutations can either be somatically acquired or inherited [51,52]. Noncanonical MPL mutations include T119I, S204F/P, and E230G in the extracellular domain and Y591D/N in the cytoplasmic domain. Noncanonical JAK2 mutations include V625F and F556V [51,52]. Functional studies of MPL and JAK2 noncanonical mutations revealed that they are weak gain-of-function mutations that activate JAK2- STAT signaling [51,52].
The known somatic driver mutations in JAK2, MPL, CALR, and CSF3R cannot fully explain the heterogeneity of MPNs. The development of next-generation sequencing has enabled the identification of several mutations that act in concert in more than one-third of MPN patients [53]. These mutations are not specific or restricted to MPN, occurring in other myeloid malignancies including acute myeloid leukemia (AML) [54,55] and myelodysplastic syndrome (MDS) [56,57], as well as in some elderly patients without an overt myeloid malignancy [58-60]. Presence of cooperating mutations in myeloid genes modifies the differentiation process and increases the myelodysplastic features of the disease, implicating continuity among MPN, MDS/MPN, and MDS [7]. The most commonly affected genes are those important in epigenetic regulation, messenger RNA splicing, transcriptional mechanisms, and signal transduction. The gene mutations are not mutually exclusive; two or more mutations can occur simultaneously [53]. The total number of mutations is inversely related to survival and the risk of leukemic transformation in MPN [53]. Cooperating driver gene mutation frequency differs by disease stage; in general, these mutations tend to be more common in patients with progressive disease [61].
The TET2 and DNMT3A proteins play a central role in the regulation of DNA methylation at cytosine guanine dinucleotide (CpG) sequences [62]. TET2 is involved in active demethylation through oxidation of 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC), a process thought to be particularly important for stem cell gene regulation [62]. DNMT3A is responsible for cytosine methylation [63]. All TET2 mutations in MPN are loss-of-function mutations and occur in approximately 10% to 20% cases across MPN subtypes [64]. DNMT3A mutations are less frequent, being present in 5% to 10% of all cases across MPN subtypes [63]. These two types of mutations increase the self-renewal of JAK2 V617F HSCs and play an important role in disease initiation [65]. They also promote disease progression when they occur as secondary mutations, but their role in myelofibrosis and leukemic transformation remains unclear [65].
Mutations in ASXL1 and EZH2, which encode factors involved in histone methylation, are overrepresented in myelofibrosis and AML-transformed disease [53,66]. Mutations in ASXL1 are the second most common epigenetic regulator mutation in MPN after TET2 [8,67]. These mutations occur in approximately 25% of PMF cases [7]. ASXL1 mutations are associated with a poor prognosis and higher frequency of leukemic transformation [68]. A recent study using transgenic mice showed that truncated ASXL1 exerts a gain-of-function effect through interaction with bromodomain and extra-terminal domain (BET) bromodomain-containing protein 4; this finding suggests a new therapeutic approach for targeting ASXL1 mutations in myeloid malignancies [69]. EZH2, a histone methyltransferase of the polycomb repressive complex 2 (PRC2), is involved in the repressive trimethylation of histone H3 at lysine 27 (H3K27) [70]. Loss-of-function mutations in EZH2 have been described in MPNs and result in the de-repression of a set of genes that includes a number of oncogenes (e.g., LMO1 and HOXA9); these mutations are associated with increased HSC self-renewal [70]. EZH2 mutations are relatively common in PMF cases (5% to 10%) and are associated with JAK2 V617F and a poor prognosis [71]. Recent knock-in mice experiments revealed that loss of Ezh2 along with the presence of JAK2 V617F inhibits erythropoiesis and promotes megakaryopoiesis, thereby accelerating the development of myelofibrosis [72].
A number of MPN mutations affect DNA and histone methylation in the HSC compartment, increasing self-renewal and blocking differentiation.
Mutations in spliceosome components, including SF3B1, SRSF2, U2AF1, and ZRSR2, are present in over half of MDS patients [73] and are also seen in MPN patients, especially in ET and PMF cases [66]. The role of these mutations in disease pathogenesis is unclear. Mutations in spliceosome components may alter the splicing of numerous pre-mRNAs and influence functionally relevant mis-spliced events [74,75]. SRSF2 encodes a member of the serine/arginine-rich splicing factor family that binds to exonic splicing enhancer sequences in mRNA [74]. Increasing evidence has shown that SFSR2 mutations affect mRNA motif recognition rather than causing loss of function [74]. Mutations of U2AF1 alter its binding preference to 3′ splice acceptor sites, thereby disrupting the splicing of genes involved in RNA processing and splicing, as well as protein translation [75].
MPN patients commonly show disrupted transcription factor gene expression. Nuclear factor erythroid 2 (NFE2) is commonly overexpressed in patients with PV, ET, and PMF [76,77]. The molecular pathophysiology of NFE overexpression is associated with JAK2 phosphorylation and JMJD1C demethylation [78]. JMJD1C, a histone demethylase and NFE2 target gene, contributes to NFE overexpression in MPNs through positive feedback. Depletion of JMJD1C selectively reduces JAK2 V617F-mediated cytokine-independent growth, suggesting a potential therapeutic benefit for MPN [78].
RAS genes (HRAS, NRAS, and KRAS) encode small GTPases with central roles in cell fate signaling pathways [79]. Oncogenic RAS mutations have been identified in a variety of hematologic malignances, such as juvenile myelomonocytic leukemia, chronic myelomonocytic leukemia, AML, and MPN [79]. A recent knock-in mouse study showed that the Kras G12D mutation drives the aggressive MPN phenotype through mediation of Sos1, a guanine nucleotide exchange factor [80,81]. The authors suggested that the survival of patients with KRAS mutations may increase with therapies that target the Sos1-oncogenic Kras interaction [81].
Most cases of MPN are sporadic, but several studies have shown familial clustering of the disease [82-84] and an increased risk of developing MPNs among the relatives of patients [85]. Common predisposing polymorphisms for sporadic MPNs include the germline JAK2 haplotype (GGCC, referred to as “46/1”) and TERT (rs2736100), which confer susceptibility to MPN [86,87]. However, these polymorphisms are relatively common, and few individuals who carry them develop MPN. Recent studies have identified other genetic polymorphisms associated with MPNs operating in diverse cellular processes, including JAK-STAT signaling (SH2B3), DNA cytosine methylation (TET2), transcriptional regulation (GFI1B, PINT), and cell cycle control (CHEK2, ATM) [83,88,89]. These observations have improved our understanding of how mutant clone expansion is influenced by heritable genetic polymorphisms present in the host’s genome [90].
The order in which somatic mutations are acquired influences the response to therapy, the biology of stem and progenitor cells, and clonal evolution in patients with MPNs [65,91]. Previous reports demonstrated the importance of mutation acquisition order in JAK2-mutated MPNs harboring concurrent TET2 or DNMT3A mutations [65]. Patients who acquired the JAK2 mutation first have a greater likelihood of presenting with PV than ET, and an increased risk of thrombosis. In contrast, TET2-first or DNMT3A-first patients are more likely to have ET, which accords with the greater self-renewal capacity seen with TET2/DNMT3A mutations compared to JAK2 mutations [65].
The most frequent mutation, JAK2 V617F, activates the three main hematopoietic cytokine receptors (EPOR, MPL, and G-CSFR) and is associated with PV, ET, and PMF. How the same mutation in JAK2 can occur with three different MPN phenotypes is an important question in MPN research. One possible explanation relates to the mutant allele burden, which is highly variable in granulocytes, with a threshold of detection ranging between 1% and 100% [34]. The mutant allele burden is usually low in ET (around 25%), higher in PV (frequently over 50%), and close to 100% in post-PV or post-ET myelofibrosis [34]. JAK2 V617F has also been detected at very low levels (less than 1%) in the healthy population [88]. JAK2 V617F is one of the most frequent mutations found in age-associated clonal hematopoiesis [58,59].
A subset of MPN patients develop secondary AML. Ten years after MPN diagnosis, the rate of leukemic transformation was 1% in ET, 4% in PV, and 20% in PMF [92]. Recent genomic studies have improved our understanding of the somatic mutations that contribute to the transformation of MPN to AML [93-96]. Somatic mutations targeting TP53 tumor-suppressing function are found in 20% to 30% of patients at leukemic transformation but are uncommon in the chronic phase of MPN [93,95]. Transition to leukemia is associated with clonal dominance and transition from mutation heterozygosity to homozygosity [93,95,96]. RUNX1 encodes a transcription factor that binds to enhancers and promoters to regulate normal hematopoiesis [97]. Various RUNX1 mutations and chromosomal aberrations are found in 10% of MPN to secondary leukemia transitions [94,98]. Other signal transduction gene mutations, such as NRAS, SH2B3, CBL, NF1, and FLT3, are associated with an increased risk of leukemic transformation and typically occur more frequently in the blast phase compared to the chronic phase of MPN [7,93,98]. The overall prognosis of patients with leukemic transformation remains quite poor, and treatment is challenging. However, recent advances in gene mutation studies of patients with leukemic transformation are expected to provide mechanistic and therapeutic insights [99].
The discovery of the molecular features of MPN has revealed novel diagnostic and prognostic markers, provided insight into MPN pathobiology, and prompted new research questions. However, there is much that remain unknown. For example, although several heterogeneous noncanonical mutations in MPL and JAK2 have been identified [51,52], studies have not determined which mutations drive the disease in a significant proportion of triple-negative MPN cases. The hereditary basis of MPNs are poorly understood and future studies should seek to identify novel disease-related germline mutations. In addition, the functional disease outcomes of known germline mutations should be clearly defined. The integration of each of these MPN-related molecular processes into clinical practice is needed to improve and refine disease diagnosis, prognosis, and therapeutics.
Acknowledgments
This study was supported by the Soonchunhyang University Research Fund and the Brain Korea (BK) 21 Plus Program.
Figure 1.
Schematic representation of the protein structure and mutation location. (A) JAK2, (B) MPL, (C) CALR, and (D) CSF3R. The circle size corresponds to the mutation frequency. bp, base pair; C domain, carboxy-terminal domain; FERM, 4.1 protein, ezrin, radixin and moesin; JH, Janus homology; N domain, amino-terminal domain; P domain, proline-rich domain; SH2, Src homology 2.
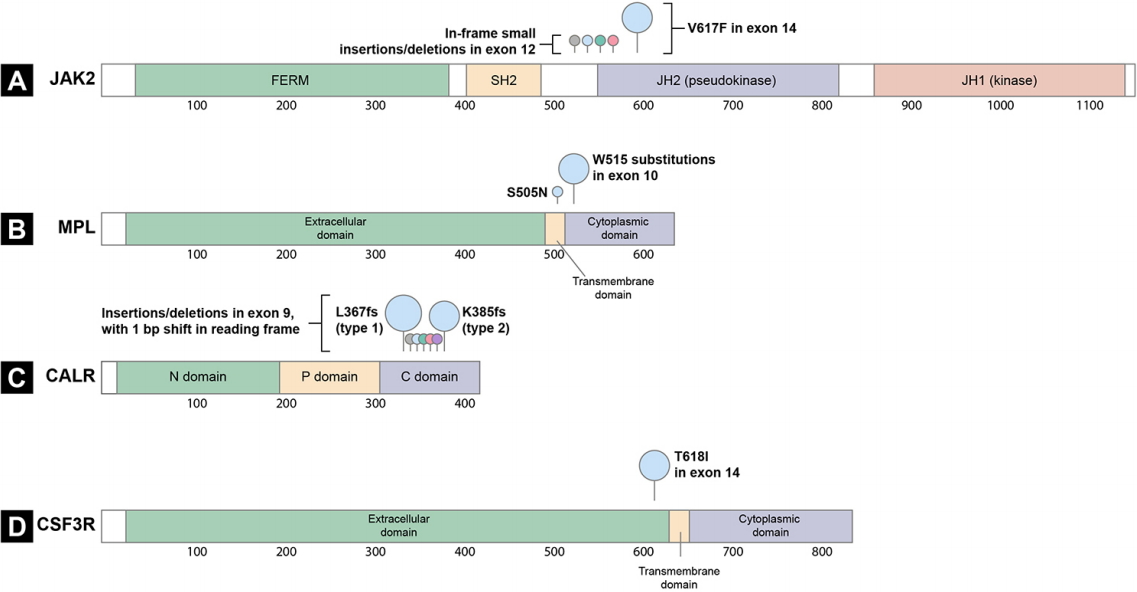
Figure 2.
Mutations in JAK2, CALR, MPL, and CSF3R lead to myeloproliferation via constitutive activation of the Janus kinase/ signal transducer and activator of transcription (JAK-STAT) pathway. (A) General mechanism of cytokine activation of the JAK-STAT pathway. Receptor binding leads to dimerization of receptor subunits, inducing transphosphorylation of JAKs. The activated JAKs phosphorylate the receptors, which are subsequently recognized by STATs. STATs are phosphorylated by JAKs and transported into the nucleus, where they regulate transcription of their target genes. Key somatic driver mutations for myeloproliferative neoplasm development occur in (B) JAK2, (C) CALR, (D) MPL, and (E) CSF3R, all of which are associated with JAK-STAT pathway activation.

REFERENCES
1. Vardiman JW. Myeloproliferative neoplasms. In: Jaffe ES, Arber DA, Campo E, Harris NL, Quintanilla-Martinez L, eds. Hematopathology. 2nd ed. Philadelphia (PA): Elsevier, 2017;847–881.
2. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon (FR): International Agency for Research on Cancer, 2017.
3. Lim Y, Lee JO, Bang SM. Incidence, survival and prevalence statistics of classical myeloproliferative neoplasm in Korea. J Korean Med Sci 2016;31:1579–1585.



4. Choi CW, Bang SM, Jang S, et al. Guidelines for the management of myeloproliferative neoplasms. Korean J Intern Med 2015;30:771–788.




5. Byun JM, Kim YJ, Youk T, Yang JJ, Yoo J, Park TS. Real world epidemiology of myeloproliferative neoplasms: a population based study in Korea 2004-2013. Ann Hematol 2017;96:373–381.



6. Dameshek W. Some speculations on the myeloproliferative syndromes [editorial]. Blood 1951;6:372–375.



7. Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017;129:667–679.



8. Grinfeld J, Nangalia J, Green AR. Molecular determinants of pathogenesis and clinical phenotype in myeloproliferative neoplasms. Haematologica 2017;102:7–17.



9. Quintas-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010;115:3109–3117.




10. James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–1148.



11. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–1061.


12. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005;352:1779–1790.


13. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–397.


14. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–3476.



15. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–2390.


16. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasm with nonmutated JAK2. N Engl J Med 2013;369:2391–2405.



17. Maxson JE, Gotlib J, Pollyea DA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med 2013;368:1781–1790.



18. Pardanani A, Lasho TL, Laborde RR, et al. CSF3R T618I is a highly prevalent and specific mutation in chronic neutrophilic leukemia. Leukemia 2013;27:1870–1873.




19. Lasho TL, Pardanani A, Tefferi A. LNK mutations in JAK2 mutation-negative erythrocytosis. N Engl J Med 2010;363:1189–1190.


20. Oh ST, Simonds EF, Jones C, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood 2010;116:988–992.




21. Grand FH, Hidalgo-Curtis CE, Ernst T, et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood 2009;113:6182–6192.



22. Schwaab J, Ernst T, Erben P, et al. Activating CBL mutations are associated with a distinct MDS/MPN phenotype. Ann Hematol 2012;91:1713–1720.



23. Suessmuth Y, Elliott J, Percy MJ, et al. A new polycythaemia vera-associated SOCS3 SH2 mutant (SOCS3F136L) cannot regulate erythropoietin responses. Br J Haematol 2009;147:450–458.



24. Lu X, Levine R, Tong W, et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci U S A 2005;102:18962–18967.



25. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–2816.



26. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–468.



27. Dusa A, Mouton C, Pecquet C, Herman M, Constantinescu SN. JAK2 V617F constitutive activation requires JH2 residue F595: a pseudokinase domain target for specific inhibitors. PLoS One 2010;5:e11157.



28. Staerk J, Lacout C, Sato T, Smith SO, Vainchenker W, Constantinescu SN. An amphipathic motif at the transmembrane-cytoplasmic junction prevents autonomous activation of the thrombopoietin receptor. Blood 2006;107:1864–1871.




29. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.



30. Defour JP, Chachoua I, Pecquet C, Constantinescu SN. Oncogenic activation of MPL/thrombopoietin receptor by 17 mutations at W515: implications for myeloproliferative neoplasms. Leukemia 2016;30:1214–1216.



31. Ding J, Komatsu H, Wakita A, et al. Familial essential thrombocythemia associated with a dominant-positive activating mutation of the c-MPL gene, which encodes for the receptor for thrombopoietin. Blood 2004;103:4198–4200.



32. Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J 2009;417:651–666.



33. Rumi E, Cazzola M. Diagnosis, risk stratification, and response evaluation in classical myeloproliferative neoplasms. Blood 2017;129:680–692.




34. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–1551.




35. Rumi E, Pietra D, Pascutto C, et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood 2014;124:1062–1069.




36. tefferi a, lasho tl, finke cm, et al. CALR vs JAK2 vs mpl-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. leukemia 2014;28:1472–1477.



37. Li J, Prins D, Park HJ, et al. Mutant calreticulin knockin mice develop thrombocytosis and myelofibrosis without a stem cell self-renewal advantage. Blood 2018;131:649–661.



38. Araki M, Yang Y, Masubuchi N, et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood 2016;127:1307–1316.



39. Chachoua I, Pecquet C, El-Khoury M, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood 2016;127:1325–1335.



40. Marty C, Pecquet C, Nivarthi H, et al. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood 2016;127:1317–1324.



41. Nivarthi H, Chen D, Cleary C, et al. Thrombopoietin receptor is required for the oncogenic function of CALR mutants. Leukemia 2016;30:1759–1763.




42. Balligand T, Achouri Y, Pecquet C, et al. Pathologic activation of thrombopoietin receptor and JAK2-STAT5 pathway by frameshift mutants of mouse calreticulin. Leukemia 2016;30:1775–1778.



43. Elf S, Abdelfattah NS, Chen E, et al. Mutant calreticulin requires both its mutant c-terminus and the thrombopoietin receptor for oncogenic transformation. Cancer Discov 2016;6:368–381.



44. Elf S, Abdelfattah NS, Baral AJ, et al. Defining the requirements for the pathogenic interaction between mutant calreticulin and MPL in MPN. Blood 2018;131:782–786.




45. Cabagnols X, Defour JP, Ugo V, et al. Differential association of calreticulin type 1 and type 2 mutations with myelofibrosis and essential thrombocytemia: relevance for disease evolution. Leukemia 2015;29:249–252.



46. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30:431–438.



47. Gotlib J, Maxson JE, George TI, Tyner JW. The new genetics of chronic neutrophilic leukemia and atypical CML: implications for diagnosis and treatment. Blood 2013;122:1707–1711.




48. Dao KH, Tyner JW. What’s different about atypical CML and chronic neutrophilic leukemia? Hematology Am Soc Hematol Educ Program 2015;2015:264–271.




49. Jang MA. Major changes to the 2017 revision of the World Health Organization classification of myeloproliferative neoplasms. Korean J Med 2018;93:351–359.


51. Cabagnols X, Favale F, Pasquier F, et al. Presence of atypical thrombopoietin receptor (MPL) mutations in triple-negative essential thrombocythemia patients. Blood 2016;127:333–342.



52. Milosevic Feenstra JD, Nivarthi H, Gisslinger H, et al. Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple-negative myeloproliferative neoplasms. Blood 2016;127:325–332.




53. Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 2014;123:2220–2228.



54. Cancer Genome Atlas Research Network; Ley TJ, et al.; Miller C. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013;368:2059–2074.



55. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 2016;374:2209–2221.



56. Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013;122:3616–3627.




57. Bejar R, Stevenson KE, Caughey B, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol 2014;32:2691–2698.



58. Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–2498.



59. McKerrell T, Park N, Moreno T, et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep 2015;10:1239–1245.



60. Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 2014;20:1472–1478.




61. Tefferi A, Lasho TL, Guglielmelli P, et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv 2016;1:21–30.




62. Zhang X, Su J, Jeong M, et al. DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat Genet 2016;48:1014–1023.




63. Stegelmann F, Bullinger L, Schlenk RF, et al. DNMT3A mutations in myeloproliferative neoplasms. Leukemia 2011;25:1217–1219.



64. Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers. N Engl J Med 2009;360:2289–2301.


65. Ortmann CA, Kent DG, Nangalia J, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med 2015;372:601–612.



66. Brecqueville M, Rey J, Bertucci F, et al. Mutation analysis of ASXL1, CBL, DNMT3A, IDH1, IDH2, JAK2, MPL, NF1, SF3B1, SUZ12, and TET2 in myeloproliferative neoplasms. Genes Chromosomes Cancer 2012;51:743–755.


67. Carbuccia N, Murati A, Trouplin V, et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia 2009;23:2183–2186.



68. Abdel-Wahab O, Tefferi A, Levine RL. Role of TET2 and ASXL1 mutations in the pathogenesis of myeloproliferative neoplasms. Hematol Oncol Clin North Am 2012;26:1053–1064.



69. Yang H, Kurtenbach S, Guo Y, et al. Gain of function of ASXL1 truncating protein in the pathogenesis of myeloid malignancies. Blood 2018;131:328–341.




70. Khan SN, Jankowska AM, Mahfouz R, et al. Multiple mechanisms deregulate EZH2 and histone H3 lysine 27 epigenetic changes in myeloid malignancies. Leukemia 2013;27:1301–1309.



71. Guglielmelli P, Biamonte F, Score J, et al. EZH2 mutational status predicts poor survival in myelofibrosis. Blood 2011;118:5227–5234.



72. Yang Y, Akada H, Nath D, Hutchison RE, Mohi G. Loss of Ezh2 cooperates with Jak2V617F in the development of myelofibrosis in a mouse model of myeloproliferative neoplasm. Blood 2016;127:3410–3423.




73. Yoshida K, Sanada M, Shiraishi Y, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011;478:64–69.



74. Kim E, Ilagan JO, Liang Y, et al. SRSF2 mutations contribute to myelodysplasia by mutant-specific effects on exon recognition. Cancer Cell 2015;27:617–630.



75. Shirai CL, Ley JN, White BS, et al. Mutant U2AF1 expression alters hematopoiesis and pre-mRNA splicing in vivo. Cancer Cell 2015;27:631–643.



76. Goerttler PS, Kreutz C, Donauer J, et al. Gene expression profiling in polycythaemia vera: overexpression of transcription factor NF-E2. Br J Haematol 2005;129:138–150.


77. Wang W, Schwemmers S, Hexner EO, Pahl HL. AML1 is overexpressed in patients with myeloproliferative neoplasms and mediates JAK2V617F-independent overexpression of NF-E2. Blood 2010;116:254–266.




78. Peeken JC, Jutzi JS, Wehrle J, et al. Epigenetic regulation of NFE2 overexpression in myeloproliferative neoplasms. Blood 2018;131:2065–2073.




79. Ward AF, Braun BS, Shannon KM. Targeting oncogenic Ras signaling in hematologic malignancies. Blood 2012;120:3397–3406.




80. Kong G, Chang YI, Damnernsawad A, et al. Loss of wildtype Kras promotes activation of all Ras isoforms in oncogenic Kras-induced leukemogenesis. Leukemia 2016;30:1542–1551.




81. You X, Kong G, Ranheim EA, Yang D, Zhou Y, Zhang J. Unique dependence on Sos1 in Kras (G12D)-induced leukemogenesis. Blood 2018;132:2575–2579.




82. Rumi E, Passamonti F, Della Porta MG, et al. Familial chronic myeloproliferative disorders: clinical phenotype and evidence of disease anticipation. J Clin Oncol 2007;25:5630–5635.


83. Rumi E, Harutyunyan AS, Pietra D, et al. LNK mutations in familial myeloproliferative neoplasms. Blood 2016;128:144–145.



84. Lanikova L, Babosova O, Swierczek S, et al. Coexistence of gain-of-function JAK2 germ line mutations with JAK2V617F in polycythemia vera. Blood 2016;128:2266–2270.




85. Landgren O, Goldin LR, Kristinsson SY, Helgadottir EA, Samuelsson J, Bjorkholm M. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood 2008;112:2199–2204.




86. Olcaydu D, Harutyunyan A, Jager R, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet 2009;41:450–454.



87. Oddsson A, Kristinsson SY, Helgason H, et al. The germline sequence variant rs2736100_C in TERT associates with myeloproliferative neoplasms. Leukemia 2014;28:1371–1374.




88. Hinds DA, Barnholt KE, Mesa RA, et al. Germ line variants predispose to both JAK2 V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood 2016;128:1121–1128.




89. Tapper W, Jones AV, Kralovics R, et al. Genetic variation at MECOM, TERT, JAK2 and HBS1L-MYB predisposes to myeloproliferative neoplasms. Nat Commun 2015;6:6691.




91. Nangalia J, Nice FL, Wedge DC, et al. DNMT3A mutations occur early or late in patients with myeloproliferative neoplasms and mutation order influences phenotype. Haematologica 2015;100:e438.



92. Cervantes F, Tassies D, Salgado C, Rovira M, Pereira A, Rozman C. Acute transformation in nonleukemic chronic myeloproliferative disorders: actuarial probability and main characteristics in a series of 218 patients. Acta Haematol 1991;85:124–127.


93. Rampal R, Ahn J, Abdel-Wahab O, et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc Natl Acad Sci U S A 2014;111:E5401–E5410.



94. Klampfl T, Harutyunyan A, Berg T, et al. Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood 2011;118:167–176.



95. Harutyunyan A, Klampfl T, Cazzola M, Kralovics R. p53 lesions in leukemic transformation. N Engl J Med 2011;364:488–490.


96. Kubesova B, Pavlova S, Malcikova J, et al. Low-burden TP53 mutations in chronic phase of myeloproliferative neoplasms: association with age, hydroxyurea administration, disease type and JAK2 mutational status. Leukemia 2018;32:450–461.



97. Sood R, Kamikubo Y, Liu P. Role of RUNX1 in hematological malignancies. Blood 2017;129:2070–2082.




-
METRICS

- Related articles
-
Guidelines for the management of myeloproliferative neoplasms2015 November;30(6)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


