 |
 |
| Korean J Intern Med > Volume 35(3); 2020 > Article |
|
See editorial "We can do much better than what we did" on page 547.
Abstract
Background/Aims
We evaluated the contemporary use of lipid-lowering therapy (LLT) in Korean patients with atherosclerotic cardiovascular disease (ASCVD), and identified factors associated with statin non-prescription.
Methods
Using the Korean Health Insurance Review and Assessment data, we identified LLT-naïve subjects newly diagnosed with ASCVD between 2011 and 2012, and followed up until 2015. LLT-naïve status was defined as no LLT prescription for 1 year before ASCVD diagnosis. ASCVD was defined as first hospitalization or emergency room visit for coronary artery disease (CAD), acute cerebrovascular disease (CVD), or peripheral artery disease (PAD). Statin intensity was defined per the 2013 American College of Cardiology/American Heart Association guideline for cholesterol treatment.
Results
The study enrolled 80,884 subjects newly diagnosed with ASCVD, of whom only 48,725 (60.2%) received LLT during the follow-up period. Statin, combination of statin and non-statin, and non-statin LLT were administered in 50.5%, 9.7%, and 0.1% of all subjects, respectively. Statins were prescribed to 80.4% of CAD patients but only to 50.2% and 46.8% of CVD and PAD patients. Statin-based LLT usually had moderate- (77.2%) or high-intensity (18.5%). Subjects not prescribed statins were younger or older (< 40 or ≥ 70 years), more commonly female, and more likely to have comorbidities. Statins were prescribed at the time of ASCVD diagnosis in 45.5% of all subjects, and in 53.0% within 90 days of diagnosis.
Atherosclerotic cardiovascular disease (ASCVD) remains one of the leading causes of death worldwide [1]. In Korea, the rates of morbidity and mortality related to ASCVD have been increasing rapidly [2,3]. As the relationship between low-density lipoprotein cholesterol (LDL-C) and ASCVD risk is well established, lowering the levels of LDL-C is considered an effective strategy for reducing ASCVD risk [4-6].
Suitable guidelines for dyslipidemia management have been established in the United States and in Europe, and are regularly being updated based on the results of clinical studies [4-8]. However, it might be difficult to apply these guidelines to clinical practice in Korea because there are racial differences in ASCVD risk and mortality, and government-issued insurance guidelines may differ in each country [1,2].
The use of lipid-lowering therapy (LLT) and the factors associated with the physicians’ decision to prescribe LLT in Korean patients with ASCVD remain unclear. In this study, we aimed to provide an overview of the current use of LLT, mainly with statins, for secondary prevention in Korean patients with ASCVD. We also evaluated the factors associated with clinical inertia in this segment of the population. We employed Korean National Health Insurance (NHI) claims data recorded between 2010 and 2015.
The Korean NHI service, which is a mandatory universal health insurance program in Korea, covers the claims of 97.0% of the Korean population, whereas the medical aid program covers those of the remaining 3%. The Health Insurance Review and Assessment (HIRA) service, which evaluates NHI claims, maintains a database with information on almost all insurance claims pertaining to the Korean population (approximately 50 million) [9].
The data included in this study were provided by the HIRA service after de-identification. Such data included age, sex, diagnosis, date of hospital visits, drug prescriptions received during inpatient and outpatient visits, hospital admissions, medical procedures, and emergency department visits. Diagnoses were coded according to the International Classification of Disease, 10th revision (ICD-10). We used data recorded between 1 August 2010 and 31 July 2015.
We identified all subjects with the first medical claims for acute coronary artery disease (CAD), acute cerebrovascular disease (CVD), or peripheral artery disease (PAD) in cohort selection period between 1 August, 2011 and 31 July, 2012. To improve the diagnostic accuracy and avoid overestimation due to inclusion of patients with previous ASCVD, we defined newly diagnosed ASCVD as an ICD-10 code corresponding to a CAD, CVD, or PAD diagnosis established following an emergency room visit or during hospitalization; the codes of relevant procedures including surgery were also used (Supplementary Table 1).
Specifically, CAD and CVD patients were identified based on medical claims mentioning acute myocardial infarction (AMI) or cerebral infarction, respectively, as the principal diagnosis or as the first additional diagnosis in patients with a different principal diagnosis. Alternatively, CAD and CVD patients were identified based on medical claims mentioning any percutaneous intervention or surgery associated with the corresponding disease; such procedures were identified using the HIRA Healthcare Common Procedure Coding System [10]. Since previous studies showed that the current diagnostic coding system is less sensitive and less specific for PAD-related illness than for CAD or CVD [11], we identified PAD patients based on claims mentioning any percutaneous intervention or surgery associated with a PAD diagnosis. Patients who required amputation of lower extremities were identified based on medical claims mentioning an associated procedure code. If a patient was diagnosed with more than one type of ASCVD on the same day during the cohort selection period, the priority for ASCVD categorization was CAD, followed by CVD, and then PAD [12].
Subjects diagnosed with ASCVD between 1 August, 2011 and 31 July, 2012 were included in this cohort, with the index date (i.e., date of diagnosis) corresponding to the cohort entry date (Fig. 1A). The cohort was followed up until 31 July, 2015, so that each subject was followed up for at least 3 years and up to 4 years. We excluded subjects with preexisting ASCVD by eliminating all cases with inpatient, outpatient, or emergency room claims for ASCVD (CAD, CVD, or PAD) during 1 year preceding the cohort entry date (pre-index period). To evaluate the pattern of LLT initiation after ASCVD diagnosis, we also excluded subjects with an LLT-related claim during the pre-index period. Therefore, the subjects included in this study were ASCVD-free for at least 1 year before the index date and were regarded as having newly diagnosed, LLT-naïve ASCVD. Furthermore, we only included adult subjects aged 20 to 89 years. Finally, we excluded subjects with unclear diagnosis of comorbidities (i.e., not in accordance with the definitions listed in Supplementary Table 2). Therefore, 80,884 subjects were enrolled in this study (Fig. 1B).
We identified the presence of comorbidities based on claims mentioning concurrent diagnoses during 1 year before cohort entry. The presence of hypertension was identified based on a combination of relevant diagnostic codes and the use of at least one anti-hypertensive drug. The presence of diabetes mellitus was identified based on at least two relevant diagnostic codes or at least one claim per year for prescription of anti-diabetic medication under diabetes diagnostic codes. The presence of heart failure, atrial fibrillation, or severe chronic kidney disease (CKD) was identified based on the corresponding ICD-10 codes (Supplementary Table 2). This approach to defining comorbidities has been validated in previous studies based on the Korean NHI cohort [10,13].
Drug exposure was assessed based on prescriptions claimed during the follow-up period from the index date. Statin use was defined in subjects who had at least one prescription for commercially available statins (rosuvastatin, pravastatin, atorvastatin, lovastatin, simvastatin, pitavastatin, or fluvastatin) between the index date and the end of the observation period (31 July, 2015). The intensity of statin therapy was classified according to the daily dose of lipid-lowering agents (LLAs), established per the cholesterol lowering guidelines issued by the American College of Cardiology (ACC) and American Heart Association (AHA): (1) high-intensity statin therapy with atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg; (2) moderate-intensity statin therapy with atorvastatin 10 to 20 mg, rosuvastatin 5 to 10 mg, simvastatin 20 to 40 mg, pravastatin 40 to 80 mg, lovastatin 40 mg, fluvastatin XL 80 mg, fluvastatin 40 mg twice a day, or pitavastatin 2 to 4 mg; (3) low-intensity statin therapy with simvastatin 10 mg, pravastatin 10 to 20 mg, lovastatin 20 mg, fluvastatin 20 to 40 mg, or pitavastatin 1 mg [6]. If a patient was prescribed more than one statin, our analysis included data from the prescription involving the highest intensity and dose. Subjects who did not have a record of statin prescription at any time during the observation period were defined as statin non-users. Subjects who did not receive a statin prescription but did receive a prescription for LLT with a non-statin LLA (ezetimibe, fibrate, omega-3, niacin, and bile acid sequestrant) were defined as non-statin LLA users.
Categorical variables are presented as frequencies and percentages, while continuous variables are presented as means ± standard deviations. We compared categorical variables using chi-square tests and continuous variables using the Student t test or analysis of variance, depending on the number of groups assessed. SAS Enterprise Guide, version 6.1 (SAS Institute Inc., Cary, NC, USA) was used to perform all statistical analyses. The significance level was set at p < 0.05.
To evaluate the factors associated with statin non-use, univariate and multivariate binary logistic regression analyses were performed. Age group, sex, ASCVD type (CAD, CVD, and PAD), and comorbidities (hypertension, hyperlipidemia, heart failure, atrial fibrillation, CKD, and diabetes), which were significantly associated with statin non-use on univariate analysis (p ≤ 0.1), were included in the multivariate logistic model. Odds ratios with 95% confidence intervals were calculated as risk estimates.
This study enrolled 80,884 subjects with newly diagnosed ASCVD and with no history of LLT during the year before the index date (Table 1). Overall, 59.3% of subjects were male and the age was 66.7 ± 13.2 years. Newly diagnosed ASCVDs included CAD (n = 26,727, 33.0%), CVD (n = 52,645, 65.1%), and PAD (n = 1,512, 1.9%). A few subjects (n = 434, 0.54%) were diagnosed with more than one type of ASCVD on the same day. Among the subjects enrolled, 41,833 (51.7%) had hypertension and 32,014 (39.6%) had diabetes.
Among the 80,884 subjects newly diagnosed with ASCVD between 2011 and 2012, 48,725 (60.2%) received LLT during the follow-up period (no later than 31 July, 2015), with most being prescribed statins. The subjects were classified according to the LLA prescribed: (1) statin-only LLT; (2) combination LLT (statin and non-statin LLAs); (3) non-statin LLT (non-statin LLAs only); (4) no LLT. Thus, statin users were defined as subjects who received statin-only or combination LLT, whereas statin non-users were defined as subjects who received non-statin LLT or no LLT at all. The proportion of subjects who received statin-only, combination, and non-statin LLT, was 50.5%, 9.7%, and 0.1%, respectively, whereas 39.8% of subjects did not receive LLT at all during the observation period after ASCVD diagnosis. Thus, the study population consisted of 60.1% statin users and 39.9% statin non-users. Among the subjects receiving LLAs, statins were used in 99.8% of cases. Among statin users (n = 48,639), 83.9% (n = 40,825) received statins only and 16.1% (n = 7,814) received combined LLT with statins and non-statin LLAs.
The use of LLT according to age, sex, underlying ASCVD, and comorbidities at baseline is illustrated in Fig. 2A and summarized in Tables 1-3. The proportion of statin users increased continuously across age groups, from 28.9% among subjects in their 20s to 70.9% among subjects in their 50s, but decreased gradually from 67.7% among subjects in their 60s to 41.3% among subjects in their 80s (p < 0.001). Statin-based LLT was most commonly prescribed among subjects aged 40 to 69 years. LLT use was less common among women, with approximately 46% of women and 35.9% of men receiving no statin therapy throughout the study period (p < 0.001). The pattern of LLT use differed also according to the type of ASCVD (p < 0.001). Specifically, statins were prescribed in 80.4% of CAD patients, which was higher than the average use of statins in the overall study population (approximately 60%), as well as higher than the use of statins among CVD and PAD patients (only 50.2% and 46.8%, respectively) (Table 3 and Fig. 2A).
Most statin users received atorvastatin (65.4%), followed by rosuvastatin (22.3%), simvastatin (8.8%), pitavastatin (4.0%), and pravastatin (3.8%). The prescriptions typically involved moderate-intensity statin therapy (77.2%), followed by high-intensity (18.5%) and low-intensity (4.2%) statin therapy.
A similar pattern of statin therapy prescription was observed in patients with CAD, CVD, and PAD. However, rosuvastatin was more frequently prescribed in CAD patients (31.8%) than in CVD and PAD patients (14.7% and 19.4%, respectively; p < 0.001), whereas atorvastatin was more frequently prescribed in CVD patients (69.9%) than in CAD and PAD patients (60.0% and 60.3%, respectively; p < 0.001). High-intensity statin therapy was less frequently prescribed in PAD patients (8.5%) than in CAD and CVD patients (19.3% and 18.2%, respectively; p < 0.001) (Fig. 2B).
Among the subjects prescribed combination LLT (9.7% of the total study population, n = 7,814), the non-statin LLA most frequently prescribed in combination with statins was ezetimibe (59.8%, n = 4,449; 9.1% of all statin users), followed by omega-3 fatty acids, fibrate, and niacin (27.3%, 23.9%, and 2.7%, respectively; n = 3,498; 7.2% of all statin users). The fibrate most frequently prescribed in combination with statins was fenofibrate (95.8%), followed by bezafibrate (3.9%). The patients who received combination LLT were younger and had a higher incidence of CAD than of CVD or PAD. Additionally, combination LLT was more frequently prescribed in subjects with diabetes (10.2% vs. 9.3% among non-diabetic subjects; p < 0.001). Fibrates were more frequently prescribed in diabetic subjects (28.4% vs. 20.7%, p < 0.0001), while ezetimibe was more frequently prescribed in non-diabetic subjects (62.1% vs. 56.5%, p < 0.0001) (Table 3 and Fig.2C).
Compared to statin users (i.e., subjects prescribed statin-only or combined LLT), statin non-users (i.e., subjects prescribed non-statin LLT or no LLT) were older, more likely to be female, less likely to have diabetes, and more likely to have heart failure, atrial fibrillation, or severe CKD (Table 1). On multivariate logistic regression analysis, factors associated with statin non-use included age (≥ 70 or < 40 years), female sex, underlying CVD or PAD, and comorbidities of heart failure, atrial fibrillation, and severe CKD. On the other hand, underlying CAD, hypertension, hyperlipidemia, and diabetes were associated with statin use (Table 2 and Fig. 3).
Among all subjects newly diagnosed with ASCVD (n = 80,884), 45.5% were prescribed statins at the time of ASCVD diagnosis, and 53.0% within 90 days of diagnosis. In patients started on statins after the index date, the median time interval from ASCVD diagnosis to the first statin prescription was 78 days (interquartile range, 15 to 506).
In this nationwide database study, we found that only 60% of LLT-naïve Koreans newly diagnosed with ASCVD were prescribed statin therapy. The use of moderate-intensity statins was favored, even for secondary prevention. We also found that adults aged < 40 or ≥ 70 years, women, and individuals diagnosed with CVD or PAD rather than CAD were less likely to receive LLT for newly diagnosed ASCVD.
Persons with established ASCVD are at very high risk for recurrent ASCVD. Secondary prevention trials indicate that statin therapy reduces the risk of recurrent cardiovascular events and mortality in persons with existing ASCVD [6,14]. Both the 2001 Adult Treatment Panel (ATP) III of the National Cholesterol Education Program and the 2nd Korean guideline for management of dyslipidemia issued by the Korean Society of Lipid and Atherosclerosis in 2003 recommended to maintain LDL-C levels ≤ 100 mg/dL in patients with coronary heart disease or related risk equivalents [2,4]. The 2004 update of the ATP III guideline and the 2008 update the Korean guideline recommended that, in very high-risk patients such as those with acute coronary syndromes, lowering LDL-C levels to ≤ 70 mg/dL should be considered [2,5]. In 2013, the ACC/AHA issued updated guidelines on the treatment of dyslipidemia, which focused on statin intensity without a target for LDL-C levels [6,15]. Thus, the latest recommendation according to the AHA/ACC guideline is that statin therapy should be initiated with high or moderate intensity in patients with clinical ASCVD including acute coronary syndromes, history of myocardial infarction (MI), stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, and PAD of atherosclerotic origin. The 3rd Korean guideline, which was issued in 2015, listed treatment algorithms involving maintenance of LDL levels according to risk factors, but recommended statin use in AMI regardless of LDL levels [2].
During the study period evaluated here (i.e., from 2011 to 2015), only 60% of patients with new-onset ASCVD and no history of statin use were started on LLT (mostly with statins). According to the guidelines currently in effect, most patients with ASCVD should be started on statins as early as possible [2,4-8]. However, it might be difficult to apply these guidelines to actual clinical practice in Korea because there are racial differences in risk factors and mortality associated with ASCVD, and because government-issued reimbursement guidelines may differ [1,2]. During the study period, both the practitioners and the national insurance programs in Korea may have been in a transition period. In 2014, the criteria of insurance coverage of LLT were revised to refer to CAD and CAD equivalent diseases with LDL-C levels ≥ 100 mg/dL, as well as to acute coronary syndrome with LDL-C levels ≥ 70 mg/dL. Despite these important updates to the guidelines and the growing evidence that statins consistently improve outcomes for the secondary prevention of ASCVD, our results indicated that statins continue to be underused, as seems to be the case worldwide [16,17]. A recent study in Korean patients reported that recommended statins are still prescribed at suboptimal rates upon discharge following MI, though the rate of statin use in this population increased gradually, from 76.9% in 2005 to 82.6% in 2011 [18].
Many studies have confirmed that early and continuous statin therapy during and after hospitalization improves the early outcomes of AMI [19]. We found that, in this study, only 53.0% of patients received their first statin prescription within 90 days after being newly diagnosed with ASCVD. Thus, to reduce the treatment gap, nationwide surveys should be conducted regularly to monitor the rate of treatment prescription and treatment intensification.
We also found that high-intensity statin therapy in particular was underused in Korean patients with established ASCVD. As documented in the 2013 ACC/AHA guidelines, as well as in the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) study [20] and the Treating to New Targets (TNT) study [21], high-intensity statin therapy is more efficacious than moderate-intensity therapy for reducing cardiovascular end points. As these results are based on studies conducted in Western populations, there is insufficient clinical evidence to support the suggestion that higher-intensity statins confer an important benefit in Korean patients with ASCVD. In a study based on the Korea AMI Registry (KAMIR), which is the first nationwide retrospective registry for AMI in Korea, high-intensity therapy did not appear superior to low-to-moderate-intensity therapy for reducing the risk of major adverse cardiac events after AMI [22]. However, other studies in Korea support the superiority of higher-intensity statin therapy to reduce adverse cardiovascular outcomes in patients with ischemic stroke or drug-eluting stents [23,24]. Further studies are warranted to clarify the benefits and adverse effects of high-intensity statin administration in Asian populations, including Koreans.
We found that women and subjects aged ≥ 70 years were less likely to be prescribed statins after being newly diagnosed with ASCVD. The 2001 and 2013 ACC/AHA guidelines do not set an upper age limit for statin treatment in patients with ASCVD and recommend that statin treatment may also be considered in older persons at higher risk [6]. A recent study conducted in the United States reported that, compared to moderate-intensity statins, high-intensity statins provided significant survival advantage in elderly patients with ASCVD [25]. Furthermore, since elderly patients have the highest absolute risk of cardiovascular disease, statin therapy is considered to be more cost-effective in this population. For these reasons, they recommend that high-intensity statin therapy should be considered in all ASCVD patients, irrespective of age.
Prior studies have indicated that, compared to men, women with cardiovascular disease are less likely to receive a statin prescription or treatment intensification for cholesterol control [17,26]. However, cardiovascular disease continues to be the leading cause of death in women worldwide [1]. Moreover, pharmacologic therapy for hyperlipidemia has been shown to be equally effective for secondary prevention in women and in men, reducing the risk of recurrent cardiac events and ASCVD mortality [26]. Therefore, to promote optimal treatment choices for ASCVD, it is necessary to increase physician and patient awareness of the benefits of LLT in women. Moreover, large-scale, long-term, adequately-powered studies are warranted to provide real-world evidence for the effect of statins in Korean populations of female and older patients with ASCVD.
We found that statin use was much lower in subjects with CVD or PAD (approximately 50%) than in those with CAD (approximately 80%). The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study showed that, in patients with stroke or transient ischemic attack, atorvastatin (80 mg/day) reduced the incidence of stroke and cardiovascular events [27]. However, SPARCL is the only trial regarding statin use for secondary prevention in stroke patients. Further large-scale randomized prospective studies are needed to support the widespread implementation of statin therapy in stroke patients.
In the present study, we found that patients with comorbidities such as heart failure and severe CKD were less likely to be prescribed statins. Two large trials, the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) and the Gruppo Italiano per lo Studio della Sopravvivenza nella Insufficienza Cardiaca-Heart Failure (the GISSI-HF) trial, failed to confirm the beneficial effect of statins in heart failure patients [28,29]. In contrast, the Chronic Heart Failure Registry and Analysis in the Tohoku District 2 (CHART‐2) results suggested that higher-intensity statin therapy has a particularly beneficial prognostic impact in heart failure patients with preserved ejection fraction in ischemic heart disease, regardless of LDL-C levels [30]. CKD carries an increased risk of cardiovascular disease [31]. The 2013 Kidney Disease: Improving Global Outcomes (KDIGO) organization guidelines recommend the use of statins in all adults ≥ 50 years with stage 1 to 4 CKD or kidney transplant, but not in those on chronic dialysis [32].
This was a retrospective observational study based on claims data from the Korean NHI database. The claims data did not include patient clinical data (e.g., physical examination results, laboratory findings) that might provide information about the severity of ASCVD and about potential statin contraindications. We did not evaluate the impact of pill burden on statin non-prescription, because our analysis data set did not include other drug prescriptions except LLT, anti-hypertensive, and anti-diabetic medications. In addition, longitudinal clinical information about interruption of LLT was not reflected in this database. Because this study was focused on the initiation of LLT after newly diagnosed ASCVD, subjects who had a record of LLA prescription at any time during the observation period were defined as LLA-users. Therefore, we did not examine LLA adherence and persistence. Moreover, we may have overestimated the use of high-intensity statins because all subjects with a prescription of high-intensity statins at any time during the observation period were defined as high-intensity statin users. Additionally, the lack of data on baseline LDL levels is a major limitation of our study. The Korean, as well as the European and the Japanese guidelines recommend treatments with specific LDL-C targets for secondary prevention of ASCVD [2,7,8,33]. Although the 2013 ACC/AHA guideline recommended immediate statin therapy regardless of LDL-C levels in very-high-risk patients with ASCVD [6], it is likely that low baseline LDL levels may at least partially explain the high rate of non-prescription of statins. However, since we did not have data regarding baseline lipid levels, it was not possible to determine whether the underuse of statins was in any way related to the LDL-C targets established by various guidelines. Further study is needed to evaluate whether baseline LDL-C levels, particularly if low, are associated with non-prescription of statin-based LLT in patients with ASCVD.
Despite these limitations, the present study provides a unique perspective into the real-world, nationwide clinical practice regarding LLT provision in Korean patients with newly detected ASCVD. We focused on analyzing the patterns of LLA prescription using longitudinal nationwide prescription data collected for at least 3 years and up to 4 years following ASCVD diagnosis. Therefore, our findings reflect the real-world clinical practice in terms of LLT prescription for secondary prevention in the general Korean population.
To summarize, our present findings indicate that, in recent years, only 60% of Korean patients with newly detected ASCVD received statin therapy. Almost 80% of CAD patients received statins, compared to only approximately 50% of CVD or PAD patients. The factors significantly associated with statin non-use were younger or older age (≤ 40 or ≥ 70 years), female sex, and underlying CVD or PAD rather than CAD. These findings, which were obtained as a result of an extensive investigation of epidemiologic data from the Korean NHI claims database, confirm the treatment gap in statin therapy in Korea and highlight the importance of continuous monitoring of real-life clinical practice. We hope that our findings will be considered when planning national public health strategies to improve guideline adherence and ensure that Korean patients with ASCVD receive appropriate statin treatment.
1. Only 60% of lipid-lowering therapy (LLT)-naïve patients newly diagnosed with atherosclerotic cardiovascular disease (ASCVD) between 2011 and 2012 in Korea received statins during the follow-up period until 2015.
2. Statins were prescribed to approximately 80% of coronary artery disease patients but only to 50% of cerebrovascular disease and peripheral artery disease patients.
3. In statin-based LLT, statin therapy was administered with moderate-intensity in approximately 80% of patients.
4. Subjects not prescribed statins were younger or older (< 40 or ≥ 70 years), more commonly female, and more likely to have comorbidities.
5. Among all subjects newly diagnosed with ASCVD, 45.5% were prescribed statins at the time of ASCVD diagnosis, and 53.0% within 90 days of diagnosis.
Supplementary Materials
Supplementary Table 1.
The definition of atherosclerotic cardiovascular disease
Conflict of Interest
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Figure 1.
Overview of the study. (A) Schematic description of the study period. (B) Patient enrollment flowchart for the study cohort. The red arrows represent the index dates in each case. ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; CVD, cerebrovascular disease; PAD, peripheral artery disease.
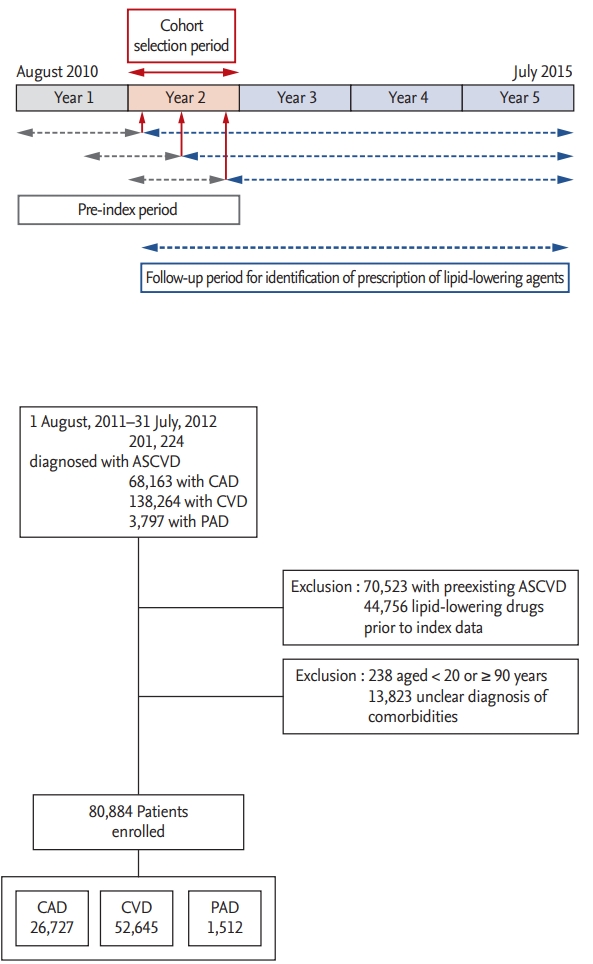
Figure 2.
Use of lipid-lowering therapy in Korean patients. (A) Use of lipid-lowering therapy for secondary prevention in patients with atherosclerotic cardiovascular disease (ASCVD). (B) Intensity of statin therapy according to the type of ASCVD. (C) Pattern of combination therapy according to underlying ASCVD and DM prevalence.CAD, coronary artery disease; CVD, cerebrovascular disease; PAD, peripheral artery disease; DM, diabetes mellitus; LLA lipid-lowering agent. a Use of a lipid-lowering agent other than statins and ezetimibe.
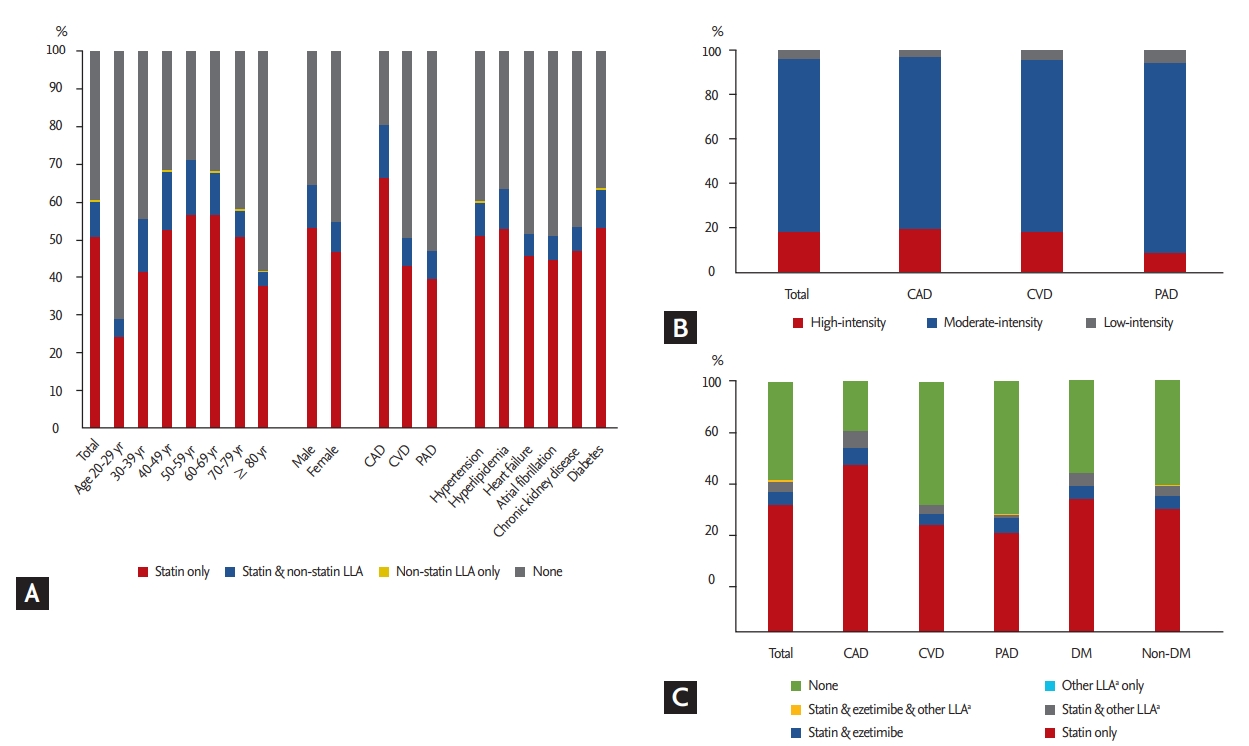
Figure 3.
Factors potentially associated with the decision not to prescribe statins. OR, odds ratio; CI, confidence in - terval; CVD, cerebrovascular disease; PAD, peripheral artery disease. a Versus CAD patients, b Versus patients aged 40 to 69 years.
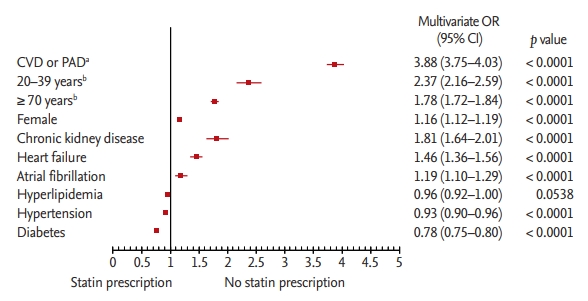
Table 1.
Baseline characteristics according to the medication of lipid-lowering agents
| Characteristic | Total (n = 80,884) | Statin only (n = 40,825, 50.5%) | Statin + other LLA (n = 7,814, 9.7%) | Other LLA (n = 86, 0.1%) | None (n = 32,159, 39.8%) | p valuea | Statin user (n = 48,639, 60.1%) | Statin nonuser (n = 32,245, 39.9%) | p valuea |
|---|---|---|---|---|---|---|---|---|---|
| Age, yr | 66.7 ± 13.2 | 65.7 ± 12.4 | 61.7 ± 12.3 | 64.0 ± 12.6 | 69.1 ± 13.9 | < 0.0001b | 65.1 ± 12.4 | 69.1 ± 13.9 | < 0.0001c |
| Age group, yr | < 0.0001 | < 0.0001 | |||||||
| 20–29 | 484 (0.6) | 116 (0.3) | 24 (0.3) | 0 | 344 (1.1) | 140 (0.3) | 344 (1.1) | ||
| 30–39 | 1,803 (2.2) | 749 (1.8) | 246 (3.1) | 3 (3.5) | 805 (2.5) | 995 (2.0) | 808 (2.5) | ||
| 40–49 | 6,877 (8.5) | 3,596 (8.8) | 1,083 (13.9) | 8 (9.3) | 2,190 (6.8) | 4,679 (9.6) | 2,198 (6.8) | ||
| 50–59 | 14,801 (18.3) | 8,365 (20.5) | 2,128 (27.2) | 18 (20.9) | 4,290 (13.3) | 10,493 (21.6) | 4,308 (13.4) | ||
| 60–69 | 17,843 (22.1) | 10,082 (24.7) | 2,001 (25.6) | 22 (25.6) | 5,738 (17.8) | 12,083 (24.8) | 5,760 (17.9) | ||
| 70–79 | 24,983 (30.9) | 12,629 (30.9) | 1,800 (23.0) | 28 (32.6) | 10,526 (32.7) | 14,429 (29.7) | 10,554 (32.7) | ||
| ≥ 80 | 14,093 (17.4) | 5,288 (13.0) | 532 (6.8) | 7 (8.1) | 8,266 (25.7) | 5,820 (12.0) | 8,273 (25.7) | ||
| Sex | < 0.0001 | < 0.0001 | |||||||
| Male | 47,970 (59.3) | 25,451 (62.3) | 5,302 (67.9) | 58 (67.4) | 17,159 (53.4) | 30,753 (63.2) | 17,217 (53.4) | ||
| Female | 32,914 (40.7) | 15,374 (37.7) | 2,512 (32.1) | 28 (32.6) | 15,000 (46.6) | 17,886 (36.8) | 15,028 (46.6) | ||
| ASCVD | < 0.0001 | < 0.0001 | |||||||
| CAD | 26,727 (33.0) | 17,672 (43.3) | 3,816 (48.8) | 10 (11.6) | 5,229 (16.3) | 21,488 (44.2) | 5,239 (16.2) | ||
| CVD | 52,645 (65.1) | 22,557 (55.3) | 3,886 (49.7) | 75 (87.2) | 26,127 (81.2) | 26,443 (54.4) | 26,202 (81.3) | ||
| PAD | 1,512 (1.9) | 596 (1.5) | 112 (1.4) | 1 (1.2) | 803 (2.5) | 708 (1.5) | 804 (2.5) | ||
| Comorbidity | |||||||||
| Hypertension | 41,833 (51.7) | 21,305 (52.2) | 3,732 (47.8) | 40 (46.5) | 16,756 (52.1) | < 0.0001 | 25,037 (51.5) | 16,796 (52.1) | 0.0873 |
| Hyperlipidemia | 14,517 (17.9) | 7,663 (18.8) | 1,509 (19.3) | 21 (24.4) | 5,324 (16.6) | < 0.0001 | 9,172 (18.9) | 5,345 (16.6) | < 0.0001 |
| Heart failure | 4,469 (5.5) | 2,040 (5.0) | 242 (3.1) | 4 (4.7) | 2,183 (6.8) | < 0.0001 | 2,282 (4.7) | 2,187 (6.8) | < 0.0001 |
| Atrial fibrillation | 3,346 (4.1) | 1,488 (3.6) | 203 (2.6) | 3 (3.5) | 1,652 (5.1) | < 0.0001 | 1,691 (3.5) | 1,655 (5.1) | < 0.0001 |
| Chronic kidney disease (≥ severe) | 1,895 (2.3) | 889 (2.2) | 117 (1.5) | 0 | 889 (2.8) | < 0.0001 | 1,006 (2.1) | 889 (2.8) | < 0.0001 |
| Diabetes | 32,014 (39.6) | 16,891 (41.4) | 3,270 (41.8) | 49 (57.0) | 11,804 (36.7) | < 0.0001 | 20,161 (41.5) | 11,853 (36.8) | < 0.0001 |
Table 2.
Univariate and multivariate logistic regression analysis of factors associated with statin non-prescription
| Variable | Statin user (n = 48,639) | Statin nonuser (n = 32,245) | Univariate OR (95% CI) | p value | Multivariatea OR (95% CI) | p value | ||
|---|---|---|---|---|---|---|---|---|
| ASCVD | < 0.0001 | < 0.0001 | ||||||
| CAD | 21,488 (44.2) | 5,239 (16.2) | 1 | (reference) | 1 | (reference) | ||
| CVD or PAD | 27,151 (55.8) | 27,006 (83.7) | 4.08 | (3.94–4.22) | 3.89 | (3.75–4.03) | ||
| Age group, yr | < 0.0001 | < 0.0001 | ||||||
| 20–39 | 1,135 (2.3) | 1,152 (3.6) | 2.26 | (2.07–2.45) | 2.37 | (2.16–2.59) | ||
| 40–69 | 27,255 (56.0) | 12,266 (38.0) | 1 | (reference) | 1 | (reference) | ||
| ≥ 70 | 20,249 (41.6) | 18,827 (58.4) | 2.07 | (2.01–2.13) | 1.78 | (1.72–1.84) | ||
| Sex | < 0.0001 | < 0.0001 | ||||||
| Male | 30,753 (63.2) | 17,217 (53.4) | 1 | (reference) | 1 | (reference) | ||
| Female | 17,886 (36.8) | 15,028 (46.6) | 1.50 | (1.46–1.54) | 1.16 | (1.12–1.19) | ||
| Comorbidities | ||||||||
| Chronic kidney disease (≥ severe) | 1,006 (2.1) | 889 (2.8) | 1.34 | (1.23–1.47) | < 0.0001 | 1.81 | (1.64–2.01) | < 0.0001 |
| Heart failure | 2,282 (4.7) | 2,187 (6.8) | 1.48 | (1.39–1.57) | < 0.0001 | 1.46 | (1.36–1.56) | < 0.0001 |
| Atrial fibrillation | 1,691 (3.5) | 1,655 (5.1) | 1.50 | (1.4–1.61) | < 0.0001 | 1.19 | (1.1–1.29) | < 0.0001 |
| Hyperlipidemia | 9,172 (18.9) | 5,345 (16.6) | 0.86 | (0.82–0.89) | < 0.0001 | 0.96 | (0.92–1.00) | 0.0538 |
| Hypertension | 25,037 (51.5) | 16,796 (52.1) | 1.02 | (1–1.05) | 0.0874 | 0.93 | (0.90–0.96) | < 0.0001 |
| Diabetes | 20,161 (41.5) | 11,853 (36.8) | 0.82 | (0.8–0.85) | < 0.0001 | 0.78 | (0.75–0.80) | < 0.0001 |
Table 3.
Status of lipid lowering treatment for secondary prevention in the patients with ASCVD, based on the type of baseline ASCVD
REFERENCES
1. Roth GA, Huffman MD, Moran AE, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015;132:1667–1678.


2. Committee for the Korean Guidelines for the Management of Dyslipidemia. 2015 Korean guidelines for the management of dyslipidemia: executive summary (English translation). Korean Circ J 2016;46:275–306.




3. Korea Centers for Disease Control and Prevention. Korea National Health and Nutrition Examination Survey. Korea Health Statistics [Internet]. Cheongju (KR): Korea Centers for Disease Control and Prevention, 2013. [cited 2019 Sep 16]. Available from: https://knhanes.cdc.go.kr/knhanes/eng/index.do.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497.


5. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227–239.


6. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–2934.


7. European Association for Cardiovascular Prevention & Rehabilitation; Reiner Z, et al.; Catapano AL. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818.



8. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058.



9. Choi NK, Chang Y, Choi YK, Hahn S, Park BJ. Signal detection of rosuvastatin compared to other statins: data-mining study using national health insurance claims database. Pharmacoepidemiol Drug Saf 2010;19:238–246.


10. Koo BK, Lee CH, Yang BR, Hwang SS, Choi NK. The incidence and prevalence of diabetes mellitus and related atherosclerotic complications in Korea: a National Health Insurance Database Study. PLoS One 2014;9:e110650.



11. Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the medicare population. Vasc Med 2008;13:209–215.


12. Subherwal S, Patel MR, Kober L, et al. Missed opportunities: despite improvement in use of cardioprotective medications among patients with lower-extremity peripheral artery disease, underuse remains. Circulation 2012;126:1345–1354.


13. Lee SR, Choi EK, Han KD, Cha MJ, Oh S, Lip GYH. Temporal trends of antithrombotic therapy for stroke prevention in Korean patients with non-valvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants: a nationwide population-based study. PLoS One 2017;12:e0189495.



14. National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421.


15. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–2236.


16. Virani SS, Woodard LD, Akeroyd JM, Ramsey DJ, Ballantyne CM, Petersen LA. Is high-intensity statin therapy associated with lower statin adherence compared with lowto moderate-intensity statin therapy? Implications of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guidelines. Clin Cardiol 2014;37:653–659.



17. Virani SS, Woodard LD, Chitwood SS, et al. Frequency and correlates of treatment intensification for elevated cholesterol levels in patients with cardiovascular disease. Am Heart J 2011;162:725–732.


18. Lee JH, Bae MH, Yang DH, et al. Contemporary trends of optimal evidence-based medical therapy at discharge for patients surviving acute myocardial infarction from the Korea acute myocardial infarction registry. Clin Cardiol 2015;38:350–356.



19. Lee CH, Lee SH, Park JS, et al. Impact of statin usage patterns on outcomes after percutaneous coronary intervention in acute myocardial infarction: Korea Working Group on Myocardial Infarction registry (KorMI) study. J Geriatr Cardiol 2014;11:93–99.


20. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–1504.


21. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–1435.


22. Kim M, Kim HK, Ahn Y, et al. Comparing high-intensity versus low-to moderate-intensity statin therapy in Korean patients with acute myocardial infarction. J Lipid Atheroscler 2014;3:97–104.

23. Kim J, Lee HS, Nam CM, Heo JH. Effects of statin intensity and adherence on the long-term prognosis after acute ischemic stroke. Stroke 2017;48:2723–2730.


24. Im E, Cho YH, Suh Y, et al. High-intensity statin treatments in clinically stable patients on aspirin monotherapy 12 months after drug-eluting stent implantation: a randomized study. Rev Esp Cardiol (Engl Ed) 2018;71:423–431.


25. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association between intensity of statin therapy and mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol 2017;2:47–54.


26. Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res 2016;118:1273–1293.



28. Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007;357:2248–2261.


29. Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1231–1239.


30. Oikawa T, Sakata Y, Nochioka K, et al. Prognostic impact of statin intensity in heart failure patients with ischemic heart disease: a report from the CHART-2 (Chronic Heart Failure Registry and Analysis in the Tohoku District 2) Study. J Am Heart Assoc 2018;7:e007524.



31. Harper CR, Jacobson TA. Managing dyslipidemia in chronic kidney disease. J Am Coll Cardiol 2008;51:2375–2384.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



