To the Editor,
Amniotic fluid embolism (AFE) or "anaphylactoid syndrome of pregnancy" was first described by Ricardo J. Meyer in 1926 [1,2]. Although on very few occasions, this potentially deadly condition affects women during labor (70%), vaginal delivery (11%), cesarean delivery (19%), or postpartum (rarely) [1,3]. AFE has also been detected after trauma, cervical laceration, abortion, amniocentesis and manual removal of the placenta [1].
In the past decades, correct diagnosis has turned out difficult and different according to diagnostic criteria adopted [4]. Only recently, uniform diagnostic criteria have been introduced to search for AFE cases, especially in its classical form [4].
Typical AFE clinical presentation includes a sudden triad of hypoxia, hypotension and coagulopathy. This triad is secondary to hemodynamic collapse, respiratory compromise and disseminated intravascular coagulation (DIC), due to amniotic fluid entering maternal circulation [3].
However, AFE can show very variable atypical forms, whose symptoms and signs differ depending on the concerned viscera and degree of involvement [5]. The diagnosis of such forms is often underestimated [5].
We introduce a very rare case of atypical AFE presentation with hepatic involvement (hepatic abscess), colic involvement (stenosing inflammation) and DIC, in the absence of classical symptoms and signs of cardiovascular and respiratory impairment, whose diagnosis has only been obtained by histopathology of surgical specimens.
A 27-year-old Moroccan woman presented to Gynecology and Obstetrics Unit for caesarean delivery due to umbilical cord prolapse and membrane breakdown at 40th week. She gave birth to a 3,176 g healthy baby boy. She had a negative medical and surgical history, without previous surgery, hemostatic disorders or spontaneous bleeding. Mother and newborn were discharged on the second day, being both in good general clinical conditions.
Three days after delivery, patient came to Emergency Department complaining of fever (body temperature 38.1°C, blood pressure 135/88 mmHg, heart rate 82 beats/min, and respiratory rate 20 breaths/min) and widespread acute abdominal pain, prevalent in the right abdominal quadrants, in absence of other symptoms and signs (nausea, vomiting, change in bowel habits). Physical examination showed generalized abdominal tenderness, marked in the right hypochondrium and right flank, with a positive Blumberg sign in these quadrants.
Urgent laboratory tests showed alterations as follows: white blood cells 24.40 × 1,000/μL, red blood cells 2.68 million/μL, hemoglobin 7.1 g/dL, hematocrit 22.2%, and platelets 147 × 1,000 μL, international normalized ratio (INR) 1.59, activated partial thromboplastin time ratio 2.18, fibrinogen 178 mg/L, C-reactive protein 33.80 mg/dL, and serum procalcitonin 64.60 mg/dL. Other relevant laboratory parameters were: bilirubin 0.3 mg/dL (range, 0.2 to 1.0), aspartate aminotransferase 21 U/L (range, 2 to 40), alanine aminotransferase 11 U/L (range, 4 to 49), gamma-glutamyl transferase 11 U/L (range, 2 to 38), and alkaline phosphatase 143 U/L (range, 90 to 360). Arterial-blood gas test showed: pH 7.38 (range, 7.35 to 7.45), pO2 94.2 mmHg (range, 80 to 100), and pCO2 37.4 mmHg (range, 35 to 45).
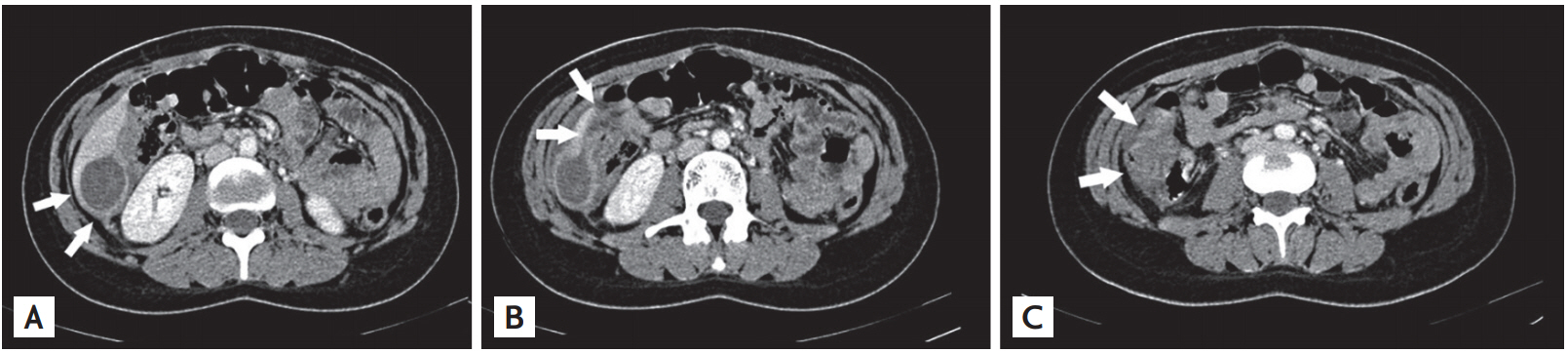
Abdomen computed tomography (CT) with intravenous contrast medium showed a subcapsular oval cystic-like 50 × 30 × 4 mm mass of hepatic segment VI with clear margins and homogeneous content. It showed strict continuity with a non-homogeneous subcapsular abscess along the lower liver margin. It was also devoid of an adipose cleavage plane with the right colon flexure appearing modestly thickened (Fig. 1).
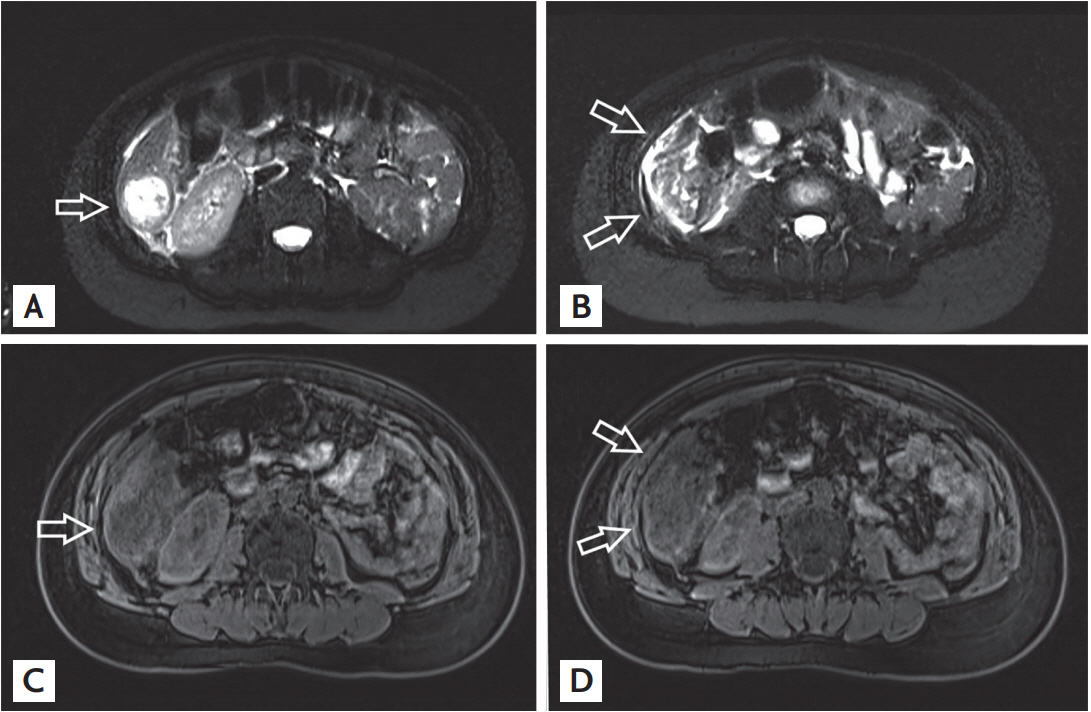
On suspicion of hepatic hydatidosis, abdominal magnetic resonance imaging was performed, which confirmed CT findings and showed an oval 5 cm maximum diameter mass with internal septations and heterogeneous signal in the T2-weighted images. Although hepatic cyst was drained, fluid cultures were totally negative for micro-organisms including Echinococcus. Search for anti-echinococcus antibodies turned out negative (Fig. 2).
During exploratory surgery, we identified right subhepatic and right pericolic abscesses, a necrosis of hepatic segment VI, a stenosing inflammation of the distal third of the right colon and of the hepatic flexure of the colon. A toilet of the abdominal cavity, a right hemicolectomy and a non-anatomical resection of the hepatic segment VI were carried out.
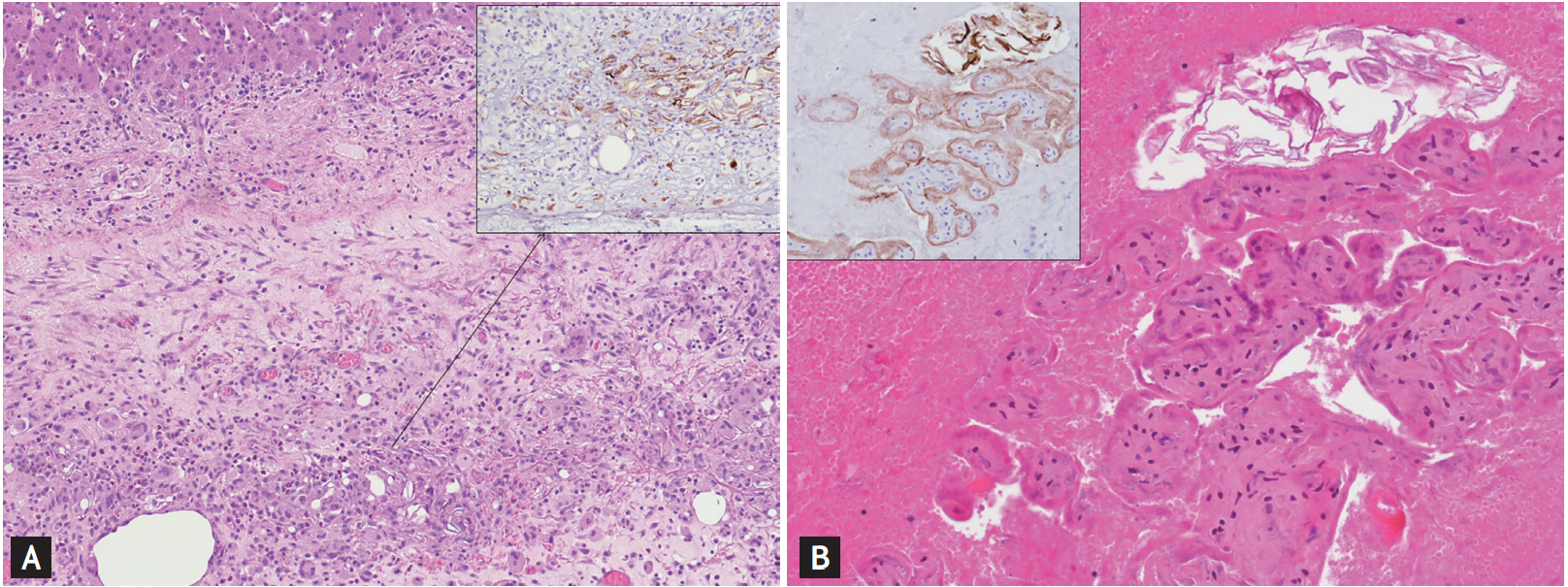
To our surprise, histological examination of hepatic parenchyma and colonic wall (Fig. 3) showed abscesses and granulomatous reaction, which was rich in eosinophilous inflammatory infiltrates, where chorionic villous and keratin debris compatible with AFE were identified. Most villi showed fibrotic-degenerative characteristics. At immunohistochemistry, outer villi layer was positively stained with high molecular weight cytokeratin (HMW-CK), programmed death 1 (PD-L1), and placental alkaline phosphatase (PLAP).
CD31 stained the endothelial cells within which keratin scales, positive for HMW-CK, are detected. The morphological characteristics were consistent with amniotic fluid and placental tissue embolism following cesarean delivery. As final histopathological examination, chest CT scan with intravenous contrast medium was performed and no anomalies turned out. The patient was discharged on the 15th post-operative day and 2 months later she is still in good clinical condition.
Due to the lack of international consensus on AFE definition and inclusion/exclusion diagnostic criteria, estimates for both AFE associated incidence and mortality rates vary significantly among countries and national registries [4]. Taking into account these important limitations, AFE incidence can be approximately estimated in 1 in 40,000 [1]. In addition, significant differences were recorded in incidence rates related to geographical variations (e.g., North America: Europe, 3:1) [1].
Combination of data from population-based studies led to a 20.4% AFE mortality rate, with a 11% to 43% mortality range [1]. AFE associated mortality rates range between 5% and 15% of all maternal deaths, about 0.5 to 1.7 deaths per 100,000 deliveries in Western countries and 1.8 to 5.9 per 100,000 deliveries in the developing countries [1]. Appropriate fatality rates and perinatal mortality account for approximately 11% to 80% and 7% to 44%, respectively [1]. Such data clearly show how remarkably such a rare condition can impact on mother’s and/or fetus’ health.
However, population-based data refer to a fundamental requirement for AFE diagnosis: cardiovascular collapse, which may occur during childbirth, labor, or within 30 minutes of post-partum [1,3,4]. Literature has reported a number of cases when the first symptom occurred later than 30 minutes after birth, as it was in our case [1,3,4]. Therefore, population-based studies do not include all AFE reported cases.
AFE pathophysiology is still unknown [1,3]. Disruption of the maternal/fetal interface with potential passage of amniotic fluid and placental material to maternal circulation could “trigger” this syndrome [3]. This could lead to abnormal activation of proinflammatory mediator systems, similar to the systemic inflammatory response syndrome [3]. Possible consequences are (1) increased levels of pulmonary vasoconstrictors (e.g., endothelin) and mechanical obstruction from cellular and acellular components of amniotic fluid, responsible for acute right ventricular failure and subsequent hemodynamic collapse; (2) acute respiratory failure, with severe hypoxemia and subsequent left ventricular failure with cardiogenic pulmonary edema and systemic hypotension; and (3) activation of factor VII and platelets with consequent DIC [3].
AFE diagnosis has clinical nature and it depends on the presence of the above clinical signs and of the exclusion of similar pathological conditions [3,4]. However, AFE can arise in atypical forms, which are often difficult to diagnose, and were classified into four sub-groups, although many cases escape an accurate locationing [5].
Our case demonstrated that AFE can show up in a complete different way from classic one, and that its consequences can have a significant impact on mother’s health, although mortality rate remains low. A clinical AFE diagnosis was impossible to be made, as symptoms and signs necessary for clinical diagnosis of the disease in its classical form were absent. It was equally impossible to include the case in one of the four specific subclasses of atypical AFE. Laboratory tests also showed no alteration of cardiorespiratory function parameters, and changes in the INR and fibrinogen levels, required for the definition of DIC, did not show any specificity.
A thorough assessment of the latest recommendations by Society for Maternal-Fetal Medicine (SMFM) and Amniotic Fluid Embolism Foundation (AFE Foundation) [3], in addition to the evidence-based reviews [5] and findings in our clinical case could lead to important statements: (1) AFE is a serious clinical syndrome that does not always occur with symptoms and signs which are typical of cardiovascular collapse; (2) AFE may affect any viscera, hollow or parenchymatous, individually and without cardiorespiratory involvement; (3) uncommon presentation arises in different ways according to the involved viscera and the degree of involvement; and (4) so, many AFE cases might escape the clinical diagnosis, due to missing classic triad.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





