INTRODUCTION
Synchronous multiple primary esophageal carcinoma (SMPEC) is defined as two or more carcinomas in different parts of the esophagus or the esophagus and other organs confirmed by pathology simultaneously or successively within 6 months [1,2]. The mechanism of SMPEC is still controversial. Nowell [3] supported the multicentric carcinogenesis theory, whereas Strong et al. [4] proposed the field carcinogenesis theory, which argues that the esophagus is in an overall cancerisation process with fields at different stages. When many pathogenic factors constantly are met, one or more carcinomas will develop in different locations simultaneously or metachronously.
Kuwabara et al. [5] examined the genetic pathways of SMPEC by microsatellite assay and discovered one or more gene alterations; the most discordant locus was TP53 (tumor protein p53), present in 11 of 29 informative cases (38%), followed by D18S61, present in 11 of 30 informative cases (37%).
There is a high prevalence of esophageal cancer in China [6], whereas the aetiology, histology, and pathogenesis differ between China and Western countries [7]. Research about SMPEC focused primarily on synchronous primary esophageal carcinoma (EC) and other primary cancers, such as head and neck, gastrointestinal tract, and lung. However, different locations in the esophagus were rarely studied. The reported incidence of SMPEC varied from 0.1% to 10.0% in the literature [8,9]. SMPEC patients are a special group of esophageal cancer patients, and should receive extra attention of clinicians. Of the 3,426 patients diagnosed with esophageal squamous cancer in the First Affiliated Hospital of China Medical University from January 2010 to December 2015, synchronous multiple primary esophageal squamous cell carcinomas (SMPESCC) patients numbered 117, representing an incidence of 3.42%.
The aim of this study is to explore the influencing factors and overall survival (OS) of SMPESCC by retrospectively reviewing the baseline clinical data of our patients. In addition, we also investigated the treatment strategies and prognosis, which may guide clinical option decisions at different clinical stages.
METHODS
Diagnostic criteria
SMPESCC was diagnosed according to the principle of Warren and Gates [2]: (1) the tumors must be clearly malignant on histologic examination; (2) the tumors must be separated by normal mucosa; and (3) the possibility that the second tumour is metastatic must be excluded. Synchronous cancer is defined as when the second primary malignancy is diagnosed within 6 months of the esophageal cancer diagnosis, and the term metachronous is used when the second primary tumor is detected more than 6 months after the first one.
Exclusion criteria
The following were our exclusion criteria: (1) the coexistence of cardiac or hypopharyngeal carcinoma; (2) an obscure boundary with the laryngopharynx or cardia; (3) existence of the precancerous lesion in two tumours; (4) carcinoma interval less than 4 cm [10]; (5) diagnosis of a hematogenous metastasis; and (6) other non-squamous cell carcinomas, including adenocarcinoma and Barrett carcinoma.
Patient characteristics
Patients with SMPESCC who had undergone treatment in the First Affiliated Hospital of China Medical University between January 2010 and December 2015 were retrospectively reviewed. In total, 117 of these patients met the diagnostic criteria. The median age of the entire study population was 59 years of age (range, 30 to 77). The tumor length was calculated by the surgical removal of the specimen or by the radiotherapy target outline. The median length of tumor was 11 cm (range, 5 to 25). The lesion interval ranged from 4 to 23 cm, and the median interval was 8 cm. There were 245 lesions altogether: 107 patients had double lesions; nine patients had triple lesions; and one patient had a quadruple lesions. These lesions were located at the cervical (n = 5), upper (n = 32), middle (n = 74), and lower (n = 134) thoracic segments. The staging of ECs was based on the TNM classification defined by the 6th Union for International Cancer Control (UICC) or American Joint Committee on Cancer (Table 1).
Treatment
Definitive surgery was performed in 52 patients: 26 patients underwent surgery alone and the other 26 were treated by definitive surgery with adjuvant radiotherapy/chemotherapy. Forty-six patients were treated by radiotherapy due to refusal of esophagectomy for personal reasons and surgical intolerance. Eighteen patients received definitive radiotherapy alone, and the remaining 28 patients were treated with concurrent/sequential chemoradiotherapy. Of the 19 patients who accepted palliative therapy, four could not fulfill the radical radiation treatment, seven were treated with radiotherapy interruption and chemotherapy, three received only chemotherapy, and the remaining five patients could tolerate only supportive therapy. The dose of definitive radiotherapy was 60 to 66 Gy. The concurrent/sequential chemotherapy consisted of two to six courses of a cisplatin-based regimen, and the median dose of interrupted radiotherapy was 24 Gy (range, 16 to 40).
Endpoint
OS was defined as the time from pathological diagnosis to death. If the patient was lost to follow-up, the last record was applied in calculating the OS; if the patient was alive by the end date of our study, the end date was adopted in the calculation. Patients alive, lost to follow-up, or who died of other diseases were all recorded as censored data.
Follow-up
Follow-up was conducted as telephone calls and outpatient clinic visits. The end date of follow-up was December 1, 2016.
Statistical analysis
Survival outcomes were calculated by the Kaplan-Meier method, and compared by the log-rank test. The Cox proportional hazards regression model was applied for multivariate survival analysis. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS version 22.0 (IBM Co., Armonk, NY, USA).
RESULTS
Survival outcome
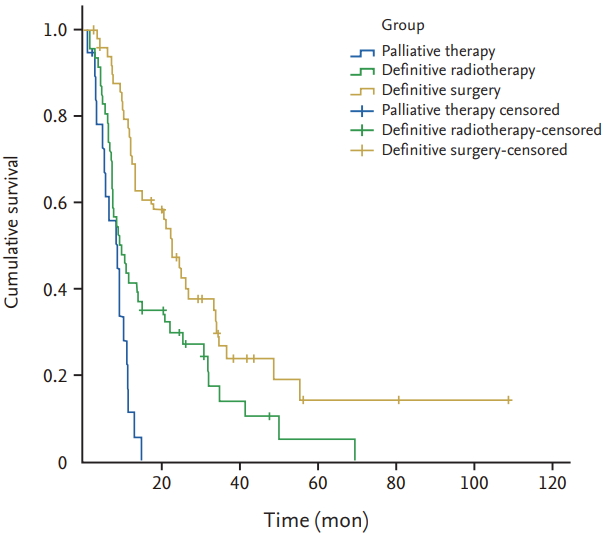
Follow-up was conducted for 117 patients (97.4%), and the median follow-up duration was 12.2 months (range, 1.1 to 117). Seventeen patients were alive at the end of follow-up, whereas 97 patients died during follow-up, and three patients were lost to follow-up. Of the patients who died, 94 died of associated carcinoma, one of cerebral haemorrhage, and two of pulmonary infections. The 1-, 2-, and 3-year OS rates were 53.8%, 30.8%, and 15.4%, respectively. The median survival time (MST) of the entire cohort was 12.5 months. The MST of patients with definitive radiotherapy, definitive surgery, and palliative therapy were 10, 24.3, and 9.3 months, respectively (Fig. 1). The MST of stage I/II patients were 34.2 and 26.7 months with definitive radiotherapy and surgery (p = 0.746); the MST of stage III and IV patients were 8.3 months versus 13.2 months, and 8 months versus 12.6 months with definitive radiotherapy and surgery, respectively (p = 0.163 and p = 0.379) (Fig. 2). The MST of patients with definitive radiotherapy was better than for those who had definitive surgery at stage I/II, whereas patients with surgery had better MST compared with those with radiotherapy at stages III and IV, although the difference did not reach statistical significance.
Prognostic analysis
Stage I/II SMPESCC patients had a significantly higher OS rate than stage III and IV patients (MST 28.8 months vs. 10 months vs. 8 months) by univariate analysis (p < 0.001). The tumour length, Karnofsky performance status (KPS), clinical M stage, and clinical stage are all prognostic factors (p = 0.027, p = 0.015, p = 0.016, and p < 0.001); the age, gender, tobacco use, alcohol consumption, family history (FH) of cancer, clinical N stage, and tumor location were not. We put factors with a p < 0.05 in univariate analysis and factors related to survival (age, gender, FH of cancer, clinical N stage, and tumor location; p < 0.2) into Cox regression analysis. Multivariate analysis showed that clinic stage (p < 0.001), FH of cancer (p = 0.032), and KPS (p = 0.013) remained independent predictors of SMPESCC. The results are displayed in Table 2.
Subgroup analysis
In the definitive radiotherapy group, the MST of concurrent/sequential chemoradiotherapy was superior to definitive radiotherapy alone (11.4 months vs. 7.9 months, p = 0.803) by univariate and multivariate analysis; in the definitive surgery group, the MST of definitive surgery alone was close to definitive surgery with adjuvant radiotherapy/chemotherapy (24 months vs. 24.4 months, p = 0.474). The statistical difference was not significant in either group.
DISCUSSION
Although esophageal adenocarcinoma (EAC) has emerged as the major type of SMPEC in some Western countries, in Asia ESCC is the predominant type and EAC remains rare [11]. Regarding histological subtypes of SMPEC in our patient data, squamous cell carcinoma accounted for the majority (94.9%), whereas eight patients had non-squamous cell carcinoma (5.1%), and their data were excluded. The male:female ratio of SMPESCC patients in our study was 109:8. This deviated largely from the gender distribution of EC patients in China (2.3 to 2.6:1) [11]. The lesions of SMPESCC patients were primarily located at the middle and lower thoracic segments (84.9%). A close ratio has been reported by Wang et al. [9] among upper gastrointestinal endoscopy detection of synchronous multiple primary cancers in the oesophagus and stomach. However, Li and Lin [12] from Tumour Hospital of Shantou University Medical College reported that the upper and middle thoracic segments accounted for 66.67% of the lesion locations. We analysed that there was a difference in amount of patients treated by surgery. From our data, 52 patients were treated by surgery accounting for 44%, which is larger than 23% (12/52) of Li and Lin [12]. As is known, middle and lower thoracic segments are easier to operate and thus more suitable for surgery. The 1- and 3-year OS rates, and the MST, in our study were 53.8%, 15.4%, and 12.5 months, respectively. In Li and Lin [12], the 1- and 3-year OS rates, and the MST, were 65.4%, 17.3%, and 15 months, respectively. The greater OS reported by Li and Lin [12] compared with our study lies primarily in the difference of the M1 and N1 stage ratio (7/52 vs. 26/117 and 26/52 vs. 60/117). In addition, both studies had small sample sizes; thus, we need larger studies to update the OS and MST of SMPEC.
The diagnosis of SMPEC was low because of a high false-negative rate of other oesophagus lesions. Histopathologically, a preoperative diagnosis of esophageal cancer is primarily conducted by fibre oesophagoscopy, whereas clinicians sometimes neglect the lower oesophagus lesion when the upper lesion is too large for the oesophagoscopy to pass through. In addition, clinicians pay less attention to SMPEC, and usually conclude with a diagnosis of single esophageal lesion. The sensitivity of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) in detecting a second primary malignancy reached 95.24% in one study. It could help oesophagoscopy detect more lesions synchronously and guide clinicians and patients in choosing the appropriate clinical therapeutic regimens, and improve prognosis [13]. However, one researcher found the sensitivity of PET alone or PET/CT was 100% for advanced-stage esophageal cancers, whereas early-stage esophageal cancers and stomach cancers were detected using Lugol chromoendoscopy but not PET [14]. Hori et al. [15] detected esophageal squamous cell carcinoma or/and head and neck squamous cell carcinoma in 1,060 patients by chromoendoscopy using iodine dye, and the results suggested that a number of Lugol-voiding lesions of 20 or more and a size of 10 mm or greater were independent risk factors for synchronous and early metachronous second primary cancer.
In our study, the tumour length and clinical M stage had statistical significance by univariate analysis, which is consistent with another report [12]. The MST of SMPESCC patients with tumour length of 11 cm or greater versus those less than 11 cm were 10 and 22 months, respectively (p = 0.027). Twenty-six patients identified with M1 by the 6th UICC TNM had supraclavicular or celiac artery lymph node metastasis at their first diagnosis, accounting for an incidence of 22.2% in the whole cohort, and they had shorter MST than other stages (MST, 8 months). In addition, surgical resection could hardly be performed on these patients because of long tumor length and high clinical M stage ratio. KPS, FH of cancer, and clinical stage were independent predictors of SMPESCC by multivariate analysis. Patients with KPS of 90 to 100 had a 63.9%, 41.7%, and 23.0% survival at 1, 2, and 3 years, respectively. The MST was 22.0 months, which was higher than the average in the whole cohort. Coia et al. [16] reported that KPS was a useful prognostic indicator for patients receiving radiation for esophageal cancer. His study showed the 2-year OS and local-regional failure of patients with KPS of 90 or 100, compared with KPS of 80 or 70, were 44% versus 32% (p = 0.0004) and 28.8% versus 49.6% (p = 0.0001) [16]. Gao et al. [17], Chinese scholars, conducted a data analysis involving 600 esophageal cancer patients to investigate the relation between FH of cancer and esophageal cancer. The result showed that young EC patients with positive FH had a poor prognosis and were inclined to genetic susceptibility [17]. In our study, patients with positive FH comprised of 26.5% of the group, and the MST was shorter compared with patients with negative FH by multivariate analysis (12.1 months vs. 14.2 months; p = 0.032; hazard ratio = 0.579). In our analysis, the MST of stage I/II SMPESCC patients was 28.8 months, compared with 10 and 8 months for stages III and IV (p < 0.001), with significant advantages. The 1-, 2-, and 3-year OS rates with stage I/II SMPESCC patients were 76.0%, 51.9%, and 27.8%, respectively; stage III and IV patients had OS rates of 34.9%, 12.7%, and 4.8%, respectively. These findings indicate that a higher diagnosis rate at the early stages of SMPESCC result in a better survival rate, especially for those with KPS of 90 or greater and who have no FH of cancer.
The reported cases of SMPEC are increasing with the advent of diagnostic techniques, whereas therapeutic strategies vary significantly. Surgery is the cornerstone in the treatment of single EC, with a MST of 13.6 to 19.3 months and a 2-year OS of 34% to 45% [18,19]. Surgical resection is the gold standard of treatment for localised esophageal cancer [20]; however, the complete resection rate decreases due to large lesions and unacceptable operative wounds in SMPEC. In our study, the MST of stage I/II patients were 34.2 and 26.7 months with definitive radiotherapy and surgery (p = 0.746); the MST of stage III patients were 8.3 and 13.2 months (p = 0.163), and 8 and 12.6 months for stage IV patients (p = 0.379). Considering the long-term survival of stage I/II patients, MST radiotherapy is preferred when definitive radiotherapy and surgery have close survival, in order to improve patients’ quality of life. Shinoto et al. [21] reported the clinical result of definitive chemoradiotherapy with a low mortality rate and acceptable morbidity for patients with synchronous head and neck carcinoma and esophageal cancer. The 2-year OS, causes-specific survival, and disease-free survival were 44%, 52%, and 33%, respectively [21]. One study reported that the 5-year OS could even reach 46.1% for SMPEC patients treated with radiotherapy [22]. The gap may be partly caused by a higher proportion of stage I/II SMPEC patients in his study (66.6%). Moreover, the study had different exclusion criteria: palliative radiotherapy, follow-up of less than 6 months, and radiation dose less than 50 Gy.
To further investigate therapeutic strategies for SMPEC patients, we conducted subgroup analyses of univariate and multivariate analysis. The MST of definitive radiotherapy or surgery alone was relatively inferior to concurrent/sequential therapy, but both subgroups had no statistical difference. Many trials support that concurrent/sequential chemoradiotherapy and definitive surgery with adjuvant radiotherapy/chemotherapy both improve EC patients’ OS effectively [23,24]. The pathogenesis and clinical characteristics of SMPEC should be further analysed based on a larger sample with prospective and randomised controlled studies.
Although we have elucidated the prognosis and influencing factors of SMPESCC, there are several limitations to this study, such as the retrospective nature of the study and selection bias. In this study, the TNM classification was applied for the staging of ECs; however, no clear SMPEC clinical stages have been authorised. Investigation for SMPEC is recommended to identify the prognosis and influencing factors further, so as to establish the standard therapeutic strategy.
In conclusion, our study showed that the overall prognosis of SMPESCC patients was poor, but primarily depended on the clinical stage itself. Stage I/II patients could achieve long-term survival through definitive treatment, especially those with KPS of 90 or 100 and a negative FH of cancer. There was no significant statistical difference in different clinical stages with patients treated by same therapeutic strategy. Definitive radiotherapy could achieve similar survival compared with definitive surgery in different clinical stages.
KEY MESSAGE
1. Synchronous multiple primary esophageal carcinoma (SMPEC) patients were a special group, which were found in 3.42% of squamous esophageal cancer patients at initial staging work-ups; therefore, it should receive extra attention of clinicians.
2. Early stage SMPEC patients could achieve longterm survival with aggressive treatment, especially those with a well performance status and who have no family history of cancer.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





