 |
 |
| Korean J Intern Med > Volume 34(5); 2019 > Article |
|
Abstract
Background/Aims
Methods
Results
Figure┬Ā1.

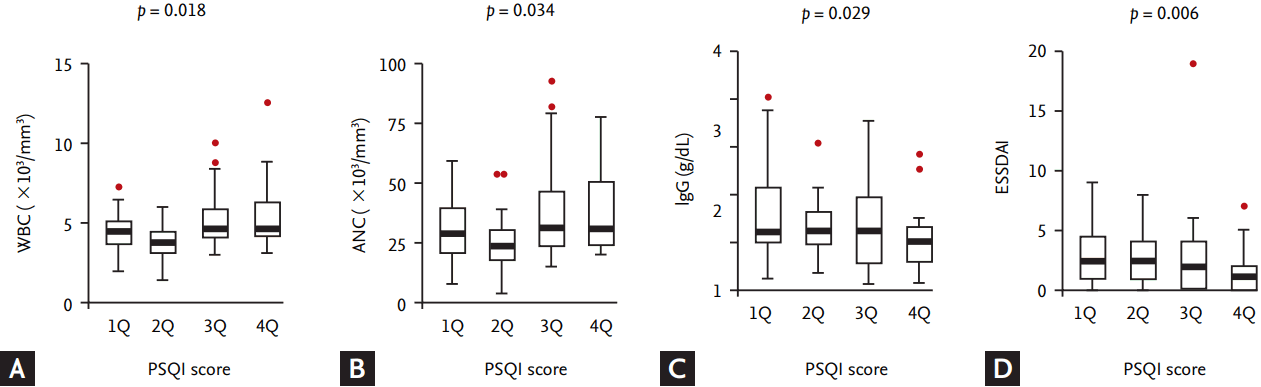
Figure┬Ā2.
Table┬Ā1.
Values are presented as number (%) or median (interquartile range). p values by the Mann-Whitney test, chi-square, or Fisher exact test.
WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ╬▓2-MG, ╬▓2-microglobulin; IgG, immunoglobulin G; ESSPRI, European League Against Rheumatism (EULAR) Sj├ČgrenŌĆÖs syndrome (SS) Patient Reported Index; ESSDAI, EULAR SS Disease Activity Index; HCQ, hydroxychloroquine; PD, prednisolone; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
Table┬Ā2.
Values are presented as median (interquartile range) or number (%). p values by the Mann-Whitney or chi-square test.
EQ-5D, EuroQol 5-dimensional questionnaire; VAS, visual analogue scale; XI, xerostomia inventory; OSDI, Ocular Surface Disease Index; ESS, European League Against Rheumatism (EULAR) sicca score; PGA, patient global assessment for Sj├ČgrenŌĆÖs syndrome (SS); PSQI, Pittsburgh Sleep Quality Index; FSS, fatigue severity score; BDI, Beck Depression Inventory.
Table┬Ā3.
p values by the Spearman rank correlation test.
EQ-5D, EuroQol 5-dimensional questionnaire; VAS, visual analogue scale; XI, xerostomia inventory; OSDI, Ocular Surface Disease Index; ESS, European League Against Rheumatism (EULAR) sicca score; PGA, patient global assessment for Sj├ČgrenŌĆÖs syndrome (SS); FSS, fatigue severity score; BDI, Beck Depression Inventory; ESSPRI, EULAR SS Patient Reported Index; WBC, white blood cell; ANC, absolute neutrophil counts; IgG, immunoglobulin G; ESSDAI, EULAR SS Disease Activity Index.
Table┬Ā4.
SE, standard error; OR, odds ratio; CI, confidence interval; PSQI, Pittsburgh Sleep Quality Index; BDI, Beck Depression Inventory; ESSPRI, European League Against Rheumatism (EULAR) Sj├ČgrenŌĆÖs syndrome (SS) Patient Reported Index; FSS, fatigue severity score; PGA, patient global assessment for SS; PD, prednisolone.
REFERENCES
-
METRICS

- Related articles
-
Lymphocytic Interstitial Pneumonia in Primary Sj├Čgren's Syndrome: A Case Report2011 March;26(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


