 |
 |
| Korean J Intern Med > Volume 33(1); 2018 > Article |
|
Abstract
Background/Aims
Methods
Results
Supplementary Materials
Supplementary┬ĀTable┬Ā1.
Supplementary┬ĀFigure┬Ā1.
Figure┬Ā1.
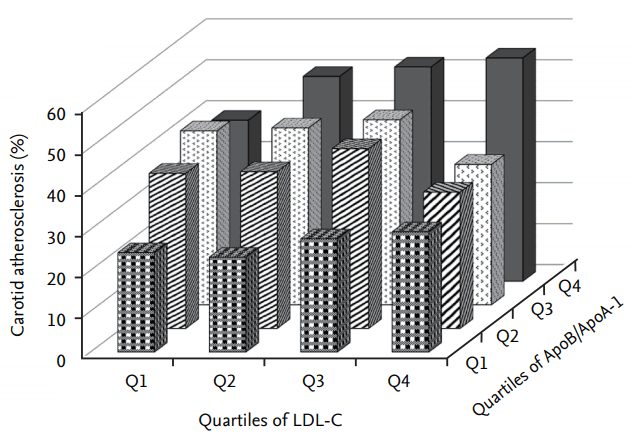
Figure┬Ā2.
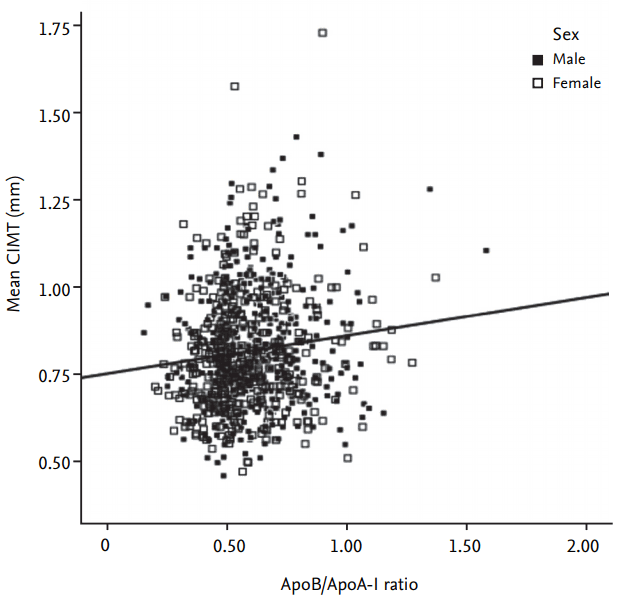
Figure┬Ā3.
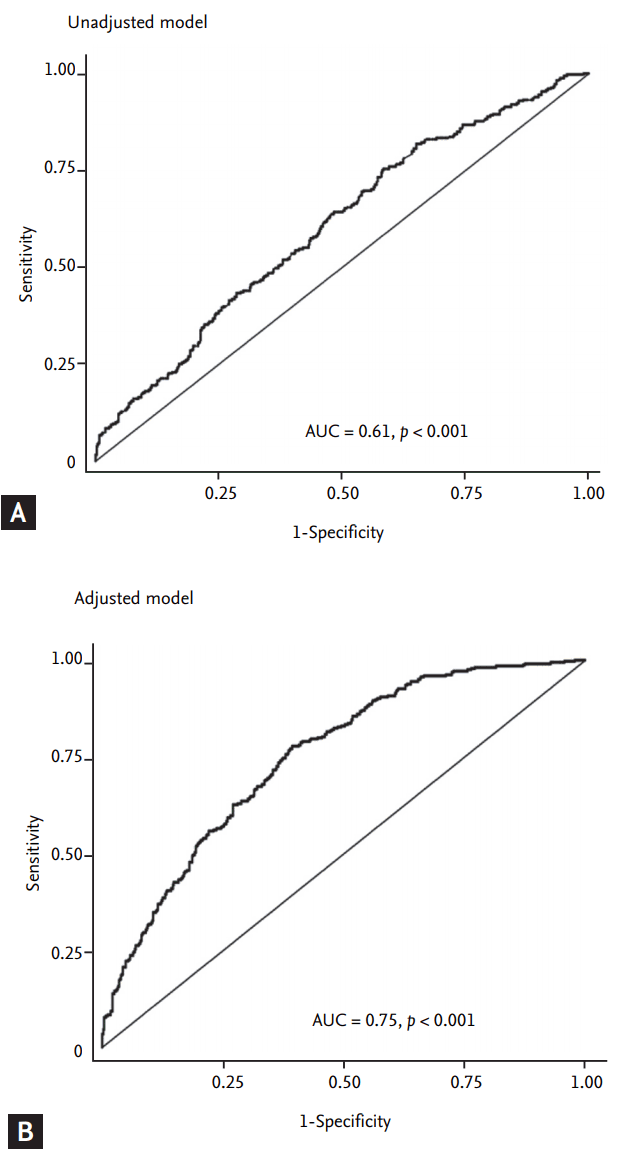
Table┬Ā1.
| Characteristic | Total |
Carotid atherosclerosisa |
p value | |
|---|---|---|---|---|
| No | Yes | |||
| No. of patients | 845 | 520 (61.7) | 325 (38.3) | |
| Age, yr | 57.2 ┬▒ 8.3 | 55.1 ┬▒ 8.0 | 60.5 ┬▒ 7.6 | < 0.001 |
| Male sex | 462 (54.7) | 268 (51.5) | 194 (59.7) | 0.021 |
| Body mass index, kg/m2 | 24.3 ┬▒ 3.2 | 24.1 ┬▒ 3.3 | 24.7 ┬▒ 3.0 | 0.009 |
| SBP, mmHg | 133.2 ┬▒ 17.3 | 131.1 ┬▒ 17.0 | 136.6 ┬▒ 17.1 | < 0.001 |
| DBP, mmHg | 85.6 ┬▒ 11.4 | 85.4 ┬▒ 11.4 | 85.8 ┬▒ 11.5 | 0.629 |
| DM duration, yr | 8.1 ┬▒ 7.3 | 7.0 ┬▒ 6.7 | 9.8 ┬▒ 7.9 | < 0.001 |
| Use of statins | 163 (19.3) | 86 (16.5) | 77 (23.7) | 0.010 |
| Use of anti-hypertensive drugs | 258 (30.5) | 139 (26.7) | 119 (36.6) | 0.002 |
| Use of insulin | 95 (11.2) | 53 (10.2) | 42 (12.9) | 0.222 |
| Use of TZD | 95 (11.2) | 52 (10.0) | 43 (13.2) | 0.148 |
| Current smokerb | 112 (24.2) | 70 (26.4) | 42 (21.3) | 0.207 |
| HbA1c, % | 7.9 ┬▒ 1.7 | 7.8 ┬▒ 1.8 | 8.0 ┬▒ 1.6 | 0.107 |
| Fasting glucose, mg/dL | 148.3 ┬▒ 54.0 | 148.0 ┬▒ 54.8 | 149.0 ┬▒ 52.8 | 0.813 |
| Total cholesterol, mg/dL | 160.2 ┬▒ 21.8 | 159.5 ┬▒ 21.9 | 161.5 ┬▒ 21.6 | 0.188 |
| Triglycerides, mg/dL | 132.9 ┬▒ 72.1 | 128.9 ┬▒ 72.1 | 139.3 ┬▒ 71.6 | 0.041 |
| HDL-C, mg/dL | 52.3 ┬▒ 15.3 | 53.6 ┬▒ 15.4 | 50.3 ┬▒ 14.9 | 0.002 |
| LDL-C, mg/dL | 84.0 (71.0ŌĆō92.0) | 83.0 (70.0ŌĆō92.0) | 86.0 (75.0ŌĆō92.0) | 0.017 |
| ApoB, mg/dL | 80.8 ┬▒ 19.5 | 78.5 ┬▒ 18.8 | 84.4 ┬▒ 20.1 | < 0.001 |
| ApoA-I, mg/dL | 138.4 ┬▒ 27.8 | 140.9 ┬▒ 28.4 | 134.4 ┬▒ 26.4 | 0.001 |
| ApoB/ApoA-I ratio | 0.61 ┬▒ 0.18 | 0.58 ┬▒ 0.17 | 0.65 ┬▒ 0.20 | < 0.001 |
| C-peptide, ng/mL | 1.8 ┬▒ 0.8 | 1.8 ┬▒ 0.8 | 1.9 ┬▒ 0.8 | 0.029 |
| Kitt, %/min | 2.2 ┬▒ 1.0 | 2.3 ┬▒ 1.0 | 2.1 ┬▒ 1.0 | 0.001 |
Values are presented as number (%), mean ┬▒ SD, or median (interquartile range).
SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; TZD, thiazolidinedione; HbA1c, glycated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; Apo, apolipoprotein; Kitt, rate constant for plasma glucose disappearance.
Table┬Ā2.
| Variable | Quartile 1 (0.16ŌĆō0.48) | Quartile 2 (0.49ŌĆō0.57) | Quartile 3 (0.58ŌĆō0.70) | Quartile 4 (0.71ŌĆō1.59) | p value |
|---|---|---|---|---|---|
| No. of patients | 211 | 211 | 214 | 209 | |
| Mean CIMTa, mm | 0.84 ┬▒ 0.14 | 0.89 ┬▒ 0.19 | 0.90 ┬▒ 0.19 | 0.93 ┬▒ 0.23 | < 0.001 |
| Presence of plaqueb | 3 (1.4) | 7 (3.3) | 6 (2.8) | 17 (8.1) | 0.001 |
| Carotid atherosclerosisc | 54 (25.6) | 81 (38.4) | 90 (42.1) | 100 (47.8) | < 0.001 |
| Age, yr | 57.6 ┬▒ 7.6 | 57.5 ┬▒ 8.3 | 56.9 ┬▒ 8.3 | 56.6 ┬▒ 8.8 | 0.545 |
| Male sex | 89 (42.2) | 111 (52.6) | 136 (63.6) | 126 (60.3) | < 0.001 |
| Body mass index, kg/m2 | 23.5 ┬▒ 3.3 | 24.0 ┬▒ 3.0 | 24.6 ┬▒ 2.9 | 25.1 ┬▒ 3.3 | < 0.001 |
| SBP, mmHg | 132.8 ┬▒ 19.0 | 132.1 ┬▒ 17.3 | 134.1 ┬▒ 17.5 | 133.9 ┬▒ 15.0 | 0.592 |
| DBP, mmHg | 85.6 ┬▒ 12.1 | 84.5 ┬▒ 11.7 | 86.2 ┬▒ 11.7 | 85.9 ┬▒ 10.1 | 0.492 |
| DM duration, yr | 7.9 ┬▒ 7.2 | 8.7 ┬▒ 7.6 | 7.5 ┬▒ 7.1 | 8.2 ┬▒ 7.5 | 0.454 |
| Use of statins | 39 (18.5) | 48 (22.7) | 41 (19.2) | 35 (16.7) | 0.472 |
| Use of anti-hypertensive drugs | 58 (27.5) | 74 (35.1) | 65 (30.4) | 61 (29.2) | 0.976 |
| Use of insulin | 32 (15.2) | 20 (9.5) | 20 (9.3) | 23 (11.0) | 0.194 |
| Use of TZD | 23 (10.9) | 30 (14.2) | 18 (8.4) | 24 (11.5) | 0.671 |
| Current smokerd | 20 (18.0) | 24 (20.3) | 32 (27.1) | 36 (31.3) | 0.009 |
| HbA1c, % | 7.9 ┬▒ 1.7 | 7.8 ┬▒ 1.6 | 7.8 ┬▒ 1.7 | 8.1 ┬▒ 1.9 | 0.288 |
| Fasting glucose, mg/dL | 146.6 ┬▒ 55.7 | 146.6 ┬▒ 48.7 | 147.4 ┬▒ 54.1 | 152.8 ┬▒ 57.3 | 0.590 |
| Total cholesterol, mg/dL | 160.9 ┬▒ 24.5 | 158.3 ┬▒ 20.9 | 159.6 ┬▒ 19.2 | 162.1 ┬▒ 22.2 | 0.305 |
| Triglycerides, mg/dL | 99.8 ┬▒ 45.2 | 125.8 ┬▒ 70.6 | 138.8 ┬▒ 70.9 | 167.3 ┬▒ 80.3 | < 0.001 |
| HDL-C, mg/dL | 65.3 ┬▒ 16.0 | 54.5 ┬▒ 11.5 | 47.9 ┬▒ 9.8 | 41.6 ┬▒ 12.0 | < 0.001 |
| LDL-C, mg/dL | 79.0 (65.0ŌĆō89.0) | 81.0 (70.0ŌĆō91.0) | 87.0 (77.0ŌĆō93.0) | 89.0 (81.0ŌĆō94.0) | < 0.001 |
| C-peptide, ng/mL | 1.6 ┬▒ 0.7 | 1.7 ┬▒ 0.8 | 1.8 ┬▒ 0.8 | 2.1 ┬▒ 1.0 | < 0.001 |
| Kitt, %/min | 2.3 ┬▒ 0.9 | 2.2 ┬▒ 1.0 | 2.2 ┬▒ 1.0 | 2.0 ┬▒ 1.0 | 0.031 |
Values are presented as mean ┬▒ SD, number (%), or median (interquartile range).
CIMT, carotid intima-media thickness; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; TZD, thiazolidinedione; HbA1c, glycated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; Kitt, rate constant for plasma glucose disappearance.
b Carotid plaque is defined as focal structures encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding intima-media thickness value, or a thickness > 1.5 mm.
Table┬Ā3.
|
Quartiles of ApoB/ApoA-I ratio |
Continuous variable OR (95% CI) | p value | |||||
|---|---|---|---|---|---|---|---|
| Quartile 1 (0.16ŌĆō0.48) | Quartile 2 (0.49ŌĆō0.57) | Quartile 3 (0.58ŌĆō0.70) | Quartile 4 (0.71ŌĆō1.59) | p for trend | |||
| Crude model | 1 (reference) | 1.81 (1.20ŌĆō2.75) | 2.11 (1.40ŌĆō3.18) | 2.67 (1.77ŌĆō4.03) | < 0.001 | 9.51 (4.32ŌĆō20.91) | < 0.001 |
| Model 1a | 1 (reference) | 1.84 (1.18ŌĆō2.85) | 2.21 (1.42ŌĆō3.43) | 3.01 (1.93ŌĆō4.67) | < 0.001 | 13.67 (5.76ŌĆō32.49) | < 0.001 |
| Model 2b | 1 (reference) | 1.75 (1.12ŌĆō2.74) | 2.12 (1.36ŌĆō3.31) | 2.76 (1.76ŌĆō4.34) | < 0.001 | 12.27 (5.01ŌĆō30.09) | < 0.001 |
| Model 3c | 1 (reference) | 1.57 (0.98ŌĆō2.53) | 1.75 (1.04ŌĆō2.93) | 2.14 (1.21ŌĆō3.79) | 0.014 | 10.05 (3.26ŌĆō30.95) | < 0.001 |
| Model 4d | 1 (reference) | 1.57 (0.98ŌĆō2.53) | 1.75 (1.04ŌĆō2.93) | 2.14 (1.21ŌĆō3.79) | 0.014 | 10.05 (3.26ŌĆō30.97) | < 0.001 |
b Model 2 was additionally adjusted for body mass index, systolic blood pressure, duration of diabetes mellitus, glycated hemoglobin, use of statins, and use of anti-hypertensive drugs.
REFERENCES
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



