 |
 |
| Korean J Intern Med > Volume 33(5); 2018 > Article |
|
Abstract
Background/Aims
To evaluate the impact of isoniazid (INH) treatment for latent tuberculosis infection (LTBI) on the development of liver function test (LFT) abnormality and the persistence of tumor necrosis factor (TNF) inhibitors in rheumatoid arthritis (RA) patients.
Methods
We retrospectively enrolled patients with RA who were treated with TNF inhibitors at a university hospital between December 2000 and November 2011. After dividing the patients into two groups based on the occurrence of LFT abnormality during follow-up, we compared demographic and clinical features between the two groups. A multivariable logistic regression analysis was performed to identify the impact of INH treatment on LFT abnormality. The impact of INH treatment on the persistence of TNF inhibitors was also evaluated with the log-rank test and the Cox-proportional hazards model.
Results
A total of 312 RA patients including 96 patients (30.9%) who took INH for LTBI were included in this analysis. Thirty-nine patients (12.5%) experienced LFT abnormalities while using TNF inhibitors. The use of INH was associated with LFT abnormalities (odds ratio, 3.01; 95% confidence interval [CI], 1.39 to 6.48) after adjusting for covariates, including methotrexate use. However, the persistence of TNF inhibitors over 5 years did not differ between patients receiving or not receiving INH treatment (49.4 vs. 54.6%, p = 0.79). INH treatment was not a risk factor for discontinuation of TNF inhibitors (hazard ratio, 1.01; 95% CI, 0.66 to 1.57).
One concern associated with tumor necrosis factor (TNF) inhibitors is the increased risk of reactivation of latent Mycobacterium tuberculosis in patients with rheumatoid arthritis (RA). Therefore, screening and treatment for latent tuberculosis infection (LTBI) is recommended before starting TNF inhibitors. There are several treatment regimens available for the treatment of LTBI. One option includes isoniazid (INH), with the 9-month regimen preferred over the 6-month regimen due to higher efficacy. In addition, the 12-dose once-weekly regimen of INH and rifapetine is recommended as an equally effective option to the standard INH 9-month daily regimen, while a 4-month regimen of rifampin can be considered for patients who cannot tolerate INH [1-3].
Some physicians have concerns about treating patients for LTBI. These concerns are generally related to the length of treatment and the potential side effects of each medication. Asymptomatic elevation of serum liver enzyme concentrations occurs in 10% to 20% of patients taking INH, though these levels usually return to normal. Clinical hepatitis occurs in about 0.1% of patients taking INH [4], which could increase if INH is combined with other hepatotoxic agents such as methotrexate (MTX) and leflunomide.
The potential hepatotoxicity of INH is of concern in RA patients treated with not only conventional disease-modifying anti-rheumatic diseases (DMARDs) [5-7] but also TNF inhibitors [8,9]. A previous report showed that half of RA patients treated with INH experienced liver function test (LFT) abnormalities and suggested that the incidence of hepatotoxicity due to INH in RA patients treated with MTX, sulfasalazine and TNF inhibitors was high [8]. Potential hepatotoxicity of INH can lead to discontinuation of concomitant MTX and it may result in reducing the effectiveness of TNF inhibitors. In addition, patients who cannot treat the LTBI may stop their TNF inhibitor. However, previous studies were performed in a small number of RA patients and the impact of INH treatment on the persistence of TNF inhibitors has not been studied.
We sought to identify the risk of INH treatment for LTBI on LFT abnormality and to evaluate the impact of INH treatment on the persistence of TNF inhibitors in RA patients.
The retrospective registry of Korean RA patients who used biologic DMARDs (REtrospective study for Safety and Effectiveness of Anti-RA treatment with biologiCs [RESEARCh]) was used to evaluate the risk of LFT abnormalities due to INH treatment for LTBI and their impact on the persistence of TNF inhibitors [10]. We identified patients who had ever taken biologic DMARDs between December 2000 and June 2011 from medical records of one university hospital, and enrolled these patients in the RESEARCh database. Comprehensive chart reviews for all patients were undertaken by well-trained health professionals. Demographics, disease activity, comorbidities, treatments, and laboratory data at the first dose of biologic DMARDs were recorded. Treatments, disease activity, and serious adverse events during treatment with biologic DMARDs were also collected.
The RESEARCh study was approved by the Institutional Review Board (HYUH IRB 2010-R-71), and informed consent was waived because the data was de-identified and collected retrospectively.
Among 442 RA patients who used TNF inhibitors, we included 312 patients who had sufficient data available on disease activity and liver enzyme concentrations. Patients who had hepatitis B or C virus (n = 11), insufficient data on LFTs or LTBI treatment (n = 39), or lack of initial disease activity data (n = 80) were excluded.
Patients were divided into two groups based on the occurrence of LFT abnormality during the use of TNF inhibitors. The mean observational period was 27.8 ┬▒ 23.1 months in patients with LFT abnormality and 23.1 ┬▒ 22.6 months in patients without LFT abnormality. We defined LFT abnormality as any elevation of alanine aminotransaminase (ALT)/aspartate aminotransaminase (AST) levels during TNF inhibitor treatment. LFT is usually monitored every 3 to 6 months after starting TNF inhibitors. To evaluate the early safety of TNF inhibitor, the data collection at 3 months of TNF inhibitor use was also performed. Patient-reported LFT abnormality was also included in the LFT abnormality group.
To compare the baseline characteristics between groups who experienced LFT abnormality and those who did not, the chi-square test was used to compare categorical variables and the Student t test was used for continuous variables. A multivariable logistic regression model was used to identify the impact of INH treatment for LTBI on the occurrence of LFT abnormality in RA patients with TNF inhibitor treatment.
A Kaplan-Meier curve and a log-rank test were used to compare the persistence rate of TNF inhibitors between patients who did and did not receive INH treatment. Cox proportional hazards analysis was used to evaluate the impact of INH treatment on the persistence of TNF inhibitors.
These statistical analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC, USA). All p values were twotailed and p < 0.05 was considered statistically significant.
Among 312 patients (595.0 person-year), 39 patients (12.5%) experienced LFT abnormality during TNF inhibitor use, while the other 273 patients did not. The duration of TNF inhibitor use was similar between the two groups, at 27.8 ┬▒ 23.1 months in patients with LFT abnormality and 23.1 ┬▒ 22.6 months in patients without LFT abnormality. In patients with LFT abnormality, the proportion of males was higher (33.3% vs. 12.1%, p < 0.01), while the mean age (49.4 ┬▒ 11.6 vs. 50.4 ┬▒ 13.5, p = 0.66), disease duration (8.2 ┬▒ 6.2 years vs. 9.0 ┬▒ 7.1 years, p = 0.55), and disease activity score-28 joints (DAS28) when starting TNF inhibitors (6.2 ┬▒ 1.1 vs. 5.9 ┬▒ 0.9, p = 0.14) were comparable between the two groups. The value of LFT was 24.5 ┬▒ 13.6 (AST) and 26.8 ┬▒ 23.3 (ALT) in patients with LFT abnormality, and 18.5 ┬▒ 9.1 (AST) and 18.1 ┬▒ 15.6 (ALT) in patients without LFT abnormality.
INH was more commonly used in patients with a history of LFT abnormality (51.3% vs. 27.8%, p < 0.01). However, MTX was more commonly used in patients without LFT abnormality (69.2% vs. 84.3%, p = 0.04), while the prevalence of NSAIDs, glucocorticoids, and the type of TNF inhibitors did not differ between the two groups (Table 1).
Unadjusted analysis revealed a significant association between INH treatment and the occurrence of LFT abnormality (odds ratio [OR], 2.73; 95% confidence interval [CI], 1.38 to 5.39). LFT abnormality before starting TNF inhibitors (OR, 2.92; 95% CI, 1.07 to 7.99) was also associated with LFT abnormality during TNF inhibitor use. INH treatment (OR, 3.01; 95% CI, 1.39 to 6.48) was significantly associated with LFT abnormality on multivariable regression analysis after adjusting for other confounding factors. High disease activity when starting TNF inhibitors (OR, 1.55; 95% CI, 1.02 to 2.37) and overweight defined by body mass index Ōēź 23.0 (OR, 1.53; 95% CI, 1.02 to 2.37) were other risk factors for the occurrence of LFT abnormality, while the concomitant use of MTX was protective (OR, 0.31; 95% CI, 0.13 to 0.77) (Table 2). Although INH treatment carried a significant risk of LFT abnormality, the severity of these abnormalities was relatively low. In patients with LFT abnormality identified via laboratory tests, elevation of ASL/ALT was mild (< 3 ├Ś upper limit of normal [ULN]) in all patients.
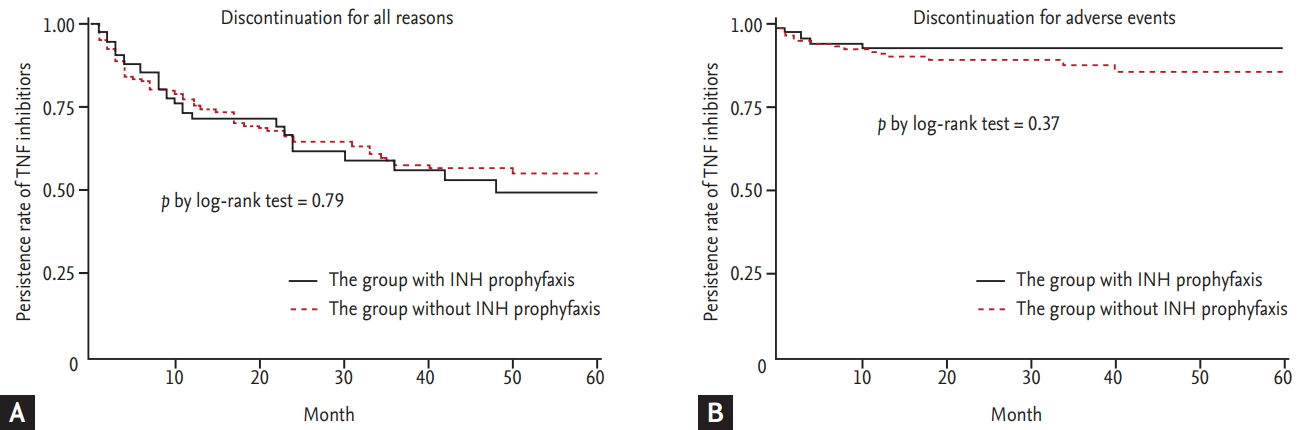
When we divided the patients according to INH treatment, 20 of 96 patients (20.8%) with INH treatment (167.1 person-year) and 19 of 216 patients (8.8%) who did not receive INH treatment (427.9 person-year) had experienced LFT abnormalities. Among patients treated with INH (n = 96), baseline characteristics of patients who experienced LFT abnormality was also compared with patients who did not experienced LFT (Supplementary Table 1). Since INH treatment was significantly associated with LFT abnormality, we further investigated the association between INH treatment and the persistence of TNF inhibitors. TNF inhibitor persistence did not differ between patients who did and did not receive INH treatment (log-rank test, p = 0.79) (Fig. 1A). When we analyzed the impact of INH treatment on the discontinuation of TNF inhibitors due to adverse events (AEs), the INH treatment group did not show a higher TNF inhibitor discontinuation rate than those in the no INH treatment group (log-rank test, p = 0.37) (Fig. 1B). Among patients treated with INH (n = 96), TNF inhibitor persistence did not differ between patients who did and did not experience LFT abnormality (Supplementary Fig. 1).
In multivariate Cox proportional hazards analysis, INH treatment for LTBI (OR, 1.01; 95% CI, 0.66 to 1.57) was not a risk factor for discontinuation of TNF inhibitors, while longer disease duration (OR, 0.96; 95% CI, 0.93 to 0.99) and a history of liver function abnormality before starting TNF inhibitors (OR, 0.32; 95% CI, 0.13 to 0.82) were protective factors for discontinuation of TNF inhibitors in RA patients (Table 3).
In this study, we found that 12.5% of RA patients who started TNF inhibitors experienced LFT abnormality during a mean observational period of 22.9 months. Although INH treatment was a risk factor for LFT abnormality, it was not a risk factor for discontinuation of TNF inhibitors. This suggests that LFT abnormality caused by INH treatment of LTBI is appropriately controlled in clinical practice.
Our study showed a slightly higher incidence of LFT abnormality in patients treated with TNF inhibitors, compared to the 5.4% in a previous report [11]. This higher rate of LFT abnormality may be explained by several reasons. Firstly, our definition of LFT abnormality was any increase in LFTs above the normal range, or a patientŌĆÖs report that they had abnormal LFTs. Since many patients had LFTs checked between each visit to the rheumatology clinic, we included patient-reported LFT abnormality in our outcomes. Secondly, the higher rate of INH treatment among Korean TNF inhibitor users was another reason for the increased rate of LFT abnormality. In our study, about 30% of TNF inhibitor users were treated with INH with starting each agent. This may be related to the higher positive rate of LTBI screening tests in Korea compared with Western countries [12-16]. Therefore, this safety issue regarding INH treatment in TNF inhibitor users is more important in countries with a higher incidence of TB or LTBI than Western countries.
Several studies have reported that INH treatment for LTBI increases liver enzyme concentrations. One study reported that among 13 RA patients with a positive LTBI test or chest X-ray, eight patients were treated with INH and four patients (50%) experienced mild to severe hepatic dysfunction. The authors suggested that the incidence of hepatotoxicity due to INH is higher in RA patients treated with MTX or sulfasalazine and TNF inhibitors [8]. On the contrary, another study reported that 11% of 44 patients who used INH combined with MTX showed transient increases in LFT, but in no case was this more than twice the ULN values. Moreover, all abnormal LFTs resolved spontaneously without intervention [9]. However, previous studies did not show the exact impact of INH treatment on the occurrence of LFT abnormality or drug persistence of TNF inhibitors because of their small sample size as well as the absence of a control group.
LFT abnormality is a common AE in RA treatment and its occurrence is dependent on not medications but on sociodemographic factors and the combined comorbidities of each patient; therefore, direct comparison between studies is quite difficult. Therefore, to identify the exact impact of INH treatment on LFT abnormality, we selected patients with some criteria from the retrospective RESEARCh database and analyzed them with a case-control design. We selected patients who started TNF inhibitors and had an available DAS28 score, because initial disease activity might influence combination treatment with MTX or other DMARDs. To identify the effect of INH treatment on LFT abnormality, we divided patients according to LFT abnormality. In multivariable analysis, INH treatment for LTBI as well as male sex and high initial disease activity were risk factors for LFT abnormality in RA patients. Interestingly, MTX was protective for the occurrence of LFT abnormality. This result can be explained by channeling bias; physicians do not prescribe MTX for patients who are at high risk for LFT abnormality. In clinical practice, MTX can be stopped for TNF users with a high risk of LFT abnormality, but the use of INH for LTBI treatment is inevitable. Therefore, in clinical practice, INH might be a stronger risk factor for LFT abnormality than MTX in RA patients who use TNF inhibitors.
In spite of the independent risk of INH treatment on the occurrence of LFT abnormality, we found that the severity of these abnormalities was relatively low. According to the US Food and Drug Administration guidance for potential drug-induced liver injury from therapeutic agents, close observation should be performed in cases with symptoms or repeat testing that shows LFT > 3 ├Ś ULN or 2-fold increases above baseline values for subjects with elevated values before drug exposure [17]. Therefore, most patients with LFT abnormality were not required to stop RA treatment or LTBI treatment, although one patient stopped TNF inhibitor and LTBI treatment due to elevated LFT.
Our second objective was to identify the impact of INH treatment on the drug persistence of TNF inhibitors in RA patients, which we investigated due to the availability of many potential clinical decisions in practice. Firstly, MTX dosage should be lowered in patients who experience elevated LFTs due to INH treatment, though this might be increase the non-response rate of TNF inhibitors. Secondly, in Korea, there are strict guidelines for INH treatment of LTBI. INH treatment must be started at least 3 weeks before starting TNF inhibitors and should be continued for 9 months after starting TNF inhibitors. If INH treatment is not tolerable for patients with LTBI, regimens of LTBI treatment or anti-RA treatment should be altered. Thirdly, adverse effects of INH treatment including LFT abnormality can affect compliance with TNF inhibitors in some patients. Therefore, we hypothesized that LFT abnormality caused by LTBI treatment can directly influence TNF inhibitor persistence.
To determine the impact of INH treatment on the persistence of TNF inhibitors, we used a cohort design. We classified patients according to INH treatment and then performed survival analysis and Cox-proportional hazards analysis. The persistence of TNF inhibitors over 5 years was similar between the groups who did and did not receive INH treatment, and INH treatment was not a risk factor for discontinuation of TNF inhibitors. Since the major reasons for discontinuation of TNF inhibitors reported in previous studies are ineffectiveness of drug and occurrence of AEs such as infections and allergic reactions, we further analyzed the impact of INH treatment on the discontinuation of TNF inhibitors due to AEs, but there was no difference between the two groups.
Our analysis has some limitations. We did not collect information about alcohol intake or herbal or alternative remedies that can potentially cause liver function abnormality. However, since the decision to begin INH is dependent on the LTBI screening test result, which is not influenced by the above personal history, we hypothesized that those baseline characteristics would be evenly controlled in the two groups by the large sample size of our study. The other limitation is that the LFT testing was not performed as regularly as it is in randomized clinical trials. However, some patients might have regular laboratory tests performed at a private clinic or hospital for their comorbid conditions. Hence, we included the patient-reported history of LFT elevation between visits to our hospital as an outcome to avoid underestimating the prevalence of LFT abnormality.
In summary, INH treatment for LTBI in RA patients who used TNF inhibitors was associated with a high incidence of LFT abnormality, but did not affect the persistence of TNF inhibitors. This result should be emphasized for TNF inhibitor users, especially in countries with high positivity of LTBI screening tests. Additional studies are warranted to determine the optimal interval of LFTs and to develop a liver function monitoring strategy.
1. This study presented the usage and safety of isoniazid (INH) treatment for latent tuberculosis infection (LTBI) in tuberculosis endemic area, Korea.
2. Patients with non-DAD-ARDS were more likely to have therapeutic alterations based on histopathologic results. However, this did not lead to a significant improvement in the mortality rate.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI16C0061).
Supplementary Materials
Supplementary┬ĀTable┬Ā1.
Comparison of demographic and clinical characteristics between rheumatoid arthritis patients treated with isoniazid with and without liver function abnormality during follow-up
Supplementary┬ĀFigure┬Ā1.
The persistence of tumor necrotizing factor (TNF) inhibitor was compared by Kaplan-Meier method. The statistical significance was evaluated with logrank test between patients who did and did not experience liver function abnormality.
Figure┬Ā1.
Kaplan-Meier curves for time to discontinuation of tumor necrotizing factor (TNF) inhibitors between patients who did and did not receive isoniazid (INH) treatment. (A) Discontinuation for all reasons. (B) Discontinuation for adverse events.
Table┬Ā1.
Comparison of demographic and clinical characteristics between rheumatoid arthritis patients with and without liver function abnormality during follow-up
| Variable | Total (n = 312) |
Liver function abnormality |
p value | |
|---|---|---|---|---|
| Yes (n = 39) | No (n = 273) | |||
| Demographics and clinical characteristics | ||||
| ŌĆāAge, yr | 50.2 ┬▒ 13.3 | 49.4 ┬▒ 11.6 | 50.4 ┬▒ 13.5 | 0.66 |
| ŌĆāFemale sex | 266 (85.3) | 26 (66.7) | 240 (87.9) | < 0.01 |
| ŌĆāDisease duration, yr | 8.9 ┬▒ 7.0 | 8.2 ┬▒ 6.2 | 9.0 ┬▒ 7.1 | 0.55 |
| ŌĆāDAS28ESR(3)a | 6.0 ┬▒ 0.9 | 6.2 ┬▒ 1.1 | 5.9 ┬▒ 0.9 | 0.14 |
| ŌĆāBMI, kg/m2 | 21.8 ┬▒ 3.8 | 22.2 ┬▒ 3.6 | 21.7 ┬▒ 3.9 | 0.42 |
| ŌĆāŌĆā< 18.5 | 49 (15.7) | 5 (12.8) | 44 (16.1) | |
| ŌĆāŌĆāŌēź 18.5 and < 23.0 | 141 (45.2) | 15 (38.5) | 126 (46.2) | |
| ŌĆāŌĆāŌēź 23.0 | 122 (39.1) | 19 (48.7) | 103 (37.7) | |
| ŌĆāAlcohol drinking (current) | 13/284 (4.6) | 2/38 (5.3) | 11/246 (4.5) | 0.69 |
| ŌĆāSmoking (current) | 14/284 (4.9) | 2/38 (5.3) | 12/246 (4.9) | |
| ŌĆāEducation, yr | 0.85 | |||
| ŌĆāŌĆāŌēż 9 | 80/265 (30.2) | 11/37 (29.7) | 69/228 (30.3) | |
| ŌĆāŌĆā10ŌĆō12 | 109/265 (41.1) | 14/37 (37.8) | 95/228 (41.7) | |
| ŌĆāŌĆā> 12 | 76/265 (28.7) | 12/37 (32.4) | 64/228 (28.1) | |
| Medication | ||||
| ŌĆāINH treatment | 96 (30.8) | 20 (51.3) | 76 (27.8) | < 0.01 |
| ŌĆāŌĆāINH treatment duration, mon | 6.7 ┬▒ 2.8 | 6.9 ┬▒ 2.5 | 6.6 ┬▒ 2.9 | 0.74 |
| ŌĆāThe no. of previous use of DMARDs | 4.1 ┬▒ 1.5 | 4.4 ┬▒ 1.6 | 4.1 ┬▒ 1.5 | 0.24 |
| ŌĆāMonths of TNF inhibitor use | 23.6 ┬▒ 22.7 | 27.8 ┬▒ 23.1 | 23.1 ┬▒ 22.6 | 0.22 |
| ŌĆāŌĆāInfliximab user | 17 (5.5) | 1 (2.6) | 16 (5.9) | 0.58 |
| ŌĆāŌĆāEtanercept user | 205 (65.7) | 28 (71.8) | 177 (64.8) | 0.58 |
| ŌĆāŌĆāAdalimumab user | 90 (28.9) | 10 (25.6) | 80 (29.3) | 0.58 |
| ŌĆāConcomitant use of corticosteroid | 263 (84.3) | 34 (87.2) | 229 (83.9) | 0.77 |
| ŌĆāConcomitant use of methotrexate | 257 (82.4) | 27 (69.2) | 230 (84.3) | 0.04 |
| ŌĆāConcomitant use of NSAIDs | 268 (85.9) | 31 (79.5) | 237 (86.8) | 0.33 |
| ŌĆāMethotrexate dose, mg/wk | 13.1 ┬▒ 3.0 | 12.7 ┬▒ 2.7 | 13.2 ┬▒ 3.0 | 0.48 |
| ŌĆāConcomitant use of acetaminophen | 64 (20.5) | 10 (25.6) | 54 (19.8) | 0.52 |
| Comorbidity | ||||
| ŌĆāPast history of hepatitis A | 1 (0.3) | - | 1 (0.4) | 1.00 |
| ŌĆāPast history of hepatitis B | 3 (1.0) | 1 (2.6) | 2 (0.7) | 0.33 |
| ŌĆāPast history of hepatitis C | 1 (0.3) | - | 1 (0.4) | 1.00 |
| ŌĆāPast history of liver abnormality | 27 (8.7) | 6 (15.4) | 21 (7.7) | 0.12 |
| ŌĆāCardiovascular disease | 7 (2.2) | - | 7 (2.6) | 0.60 |
| ŌĆāPulmonary disease | 14 (4.5) | 3 (7.7) | 11 (4.0) | 0.54 |
| ŌĆāGastrointestinal disease | 129 (41.4) | 17 (43.6) | 112 (41.0) | 0.90 |
| ŌĆāDiabetes mellitus | 35 (11.2) | 4 (10.3) | 31 (11.4) | 1.00 |
| ŌĆāMalignancy | 14 (4.5) | 2 (5.1) | 12 (4.4) | 0.69 |
| ŌĆāHypertension | 77 (24.7) | 10 (25.6) | 67 (24.5) | 1.00 |
Table┬Ā2.
The impact of INH treatment on the occurrence of liver function abnormality
Table┬Ā3.
The impact of INH treatment on the persistence of TNF inhibitorsa
REFERENCES
1. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000;161(4 Pt 2):S221ŌĆōS247.


2. Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax 1998;53:536ŌĆō548.



3. Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011;60:1650ŌĆō1653.

4. Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis clinic. JAMA 1999;281:1014ŌĆō1018.


5. Kremer JM, Furst DE, Weinblatt ME, Blotner SD. Significant changes in serum AST across hepatic histological biopsy grades: prospective analysis of 3 cohorts receiving methotrexate therapy for rheumatoid arthritis. J Rheumatol 1996;23:459ŌĆō461.

6. Siva C, Eisen SA, Shepherd R, et al. Leflunomide use during the first 33 months after food and drug administration approval: experience with a national cohort of 3,325 patients. Arthritis Rheum 2003;49:745ŌĆō751.


7. Kalden JR, Schattenkirchner M, Sorensen H, et al. The efficacy and safety of leflunomide in patients with active rheumatoid arthritis: a five-year followup study. Arthritis Rheum 2003;48:1513ŌĆō1520.


8. Vanhoof J, Landewe S, Van Wijngaerden E, Geusens P. High incidence of hepatotoxicity of isoniazid treatment for tuberculosis chemoprophylaxis in patients with rheumatoid arthritis treated with methotrexate or sulfasalazine and anti-tumour necrosis factor inhibitors. Ann Rheum Dis 2003;62:1241ŌĆō1242.



9. Mor A, Bingham CO 3rd, Kishimoto M, et al. Methotrexate combined with isoniazid treatment for latent tuberculosis is well tolerated in patients with rheumatoid arthritis: experience from an urban arthritis clinic. Ann Rheum Dis 2008;67:462ŌĆō465.


10. Cho SK, Sung YK, Choi CB, Uhm WS, Kim TH, Jun JB, et al. Treatment persistence with TNF blocker in Korean rheumatoid arthritis patients. J Rheum Dis 2011;18:161ŌĆō167.

11. Sokolove J, Strand V, Greenberg JD, et al. Risk of elevated liver enzymes associated with TNF inhibitor utilisation in patients with rheumatoid arthritis. Ann Rheum Dis 2010;69:1612ŌĆō1617.


12. Kim JH, Cho SK, Han M, et al. Factors influencing discrepancies between the QuantiFERON-TB gold in tube test and the tuberculin skin test in Korean patients with rheumatic diseases. Semin Arthritis Rheum 2013;42:424ŌĆō432.


13. Marques CD, Duarte AL, de Lorena VM, et al. Evaluation of an interferon gamma assay in the diagnosis of latent tuberculosis infection in patients with rheumatoid arthritis. Rheumatol Int 2009;30:57ŌĆō62.


14. Mariette X, Baron G, Tubach F, et al. Influence of replacing tuberculin skin test with ex vivo interferon ╬│ release assays on decision to administer prophylactic antituberculosis antibiotics before anti-TNF therapy. Ann Rheum Dis 2012;71:1783ŌĆō1790.


15. Martin J, Walsh C, Gibbs A, et al. Comparison of interferon {gamma} release assays and conventional screening tests before tumour necrosis factor {alpha} blockade in patients with inflammatory arthritis. Ann Rheum Dis 2010;69:181ŌĆō185.


16. Scrivo R, Sauzullo I, Mengoni F, et al. Serial interferon-╬│ release assays for screening and monitoring of tuberculosis infection during treatment with biologic agents. Clin Rheumatol 2012;31:1567ŌĆō1575.


17. U.S. Department of Health and Human Services Food and Drug Administration. Guidance for industry drug-induced liver injury: premarketing clinical evaluation [Internet]. Silver Spring (MD): Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration. 2009. [cited 2017 Feb 21]. Available from: http://www.fda.gov/downloads/Drugs/.../Guidances/UCM174090.pdf.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



