To the Editor,
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors originating from the gastrointestinal tract. GISTs are frequently associated with an activating, gain-offunction mutation in KIT or platelet-derived growth factor A (PDGFRA), resulting in ligand-independent activation of tyrosine-kinase [1]. The most frequent mutation occurs in KIT exon 11 (60% to 70%), followed by KIT exon 9 (5% to 10%), PDGFRA exon 18 (8%), and PDGFRA exon 12 (2%). The presence of specific mutations is clinically important because it can predict response and prognosis to tyrosine kinase inhibitors [2]. Generally, the exon 11 mutation of KIT is sensitive to imatinib mesylate, while the mutation in exon 9 is less sensitive and so requires a higher dosage. Other mutations including KIT exon 13 and 17 and PDGFRA exon 18 are resistant to imatinib. The overall prognostic role of mutational status is still controversial, but a deletion involving codons 557 and 558 of exon 11 are more aggressive compared to other exon 11 mutations, and PDGFRA mutant GISTs tend to show a slow clinical course.
Mutational analyses are usually performed at one site showing one specific mutation. However, there are some reports of two or more mutations at the same or different sites in GIST patients [3]. We report a case harboring different two KIT mutations at the primary (exon 11 mutation) and metastatic sites (exon 10 mutation), which show distinct responses to imatinib.
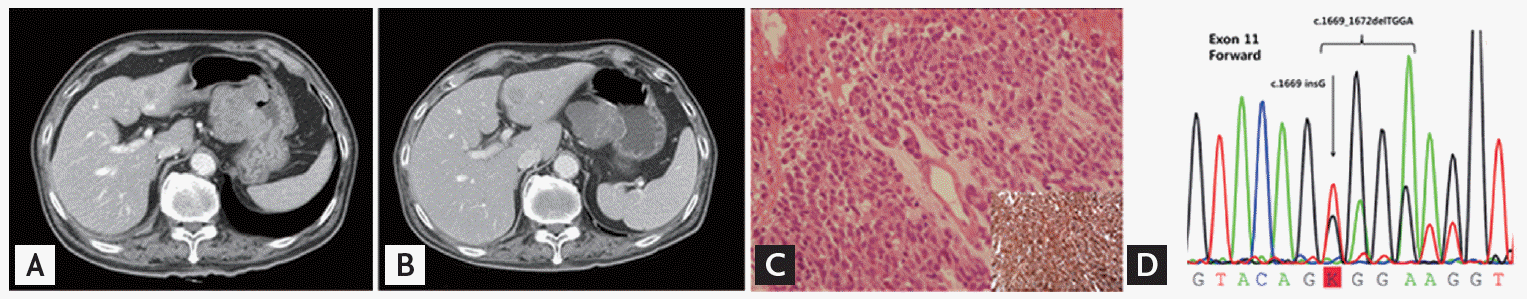
A 74-year-old male visited our hospital with dyspnea and general weakness. He had undergone video-assisted thoracoscopic lung wedge resection, bullectomy, and pleural abrasion seven years prior due to multiple bullae and recurrent pneumothorax. He had a 15 pack-year smoking history and a 1 bottle/week alcohol drinking history but quit both after the surgery. He had been on medication for diabetes mellitus and hypertension for the previous 2 years. Chest X-ray showed lung nodules, and computed tomography (CT) scan showed an incidental large exophytic mass from the stomach, with multiple lung nodules and a hepatic mass, which suggested malignancy originating from the stomach and spreading to multiple metastases (Figs. 1A and 3A). On esophagogastroduodenoscopy, a 4 ├Ś 4-cmsized fungating mass with central ulceration was found on the lesser curvature of the stomach, and histologic analysis confirmed a CD117-positive malignant GIST with epithelioid cell morphology arising from the stomach (Fig. 1C). The pathologic nature of GIST cells showed a mixed pattern of spindle and epithelioid cells (Fig. 2). Immunohistochemistry of CA117 and CD34 was strongly positive. KIT mutation analysis with Sanger direct sequencing revealed exon 11 deletion/insertion involving codons 557 and 558 (Fig. 1D). After testing, the patient was started an imatinib treatment at a dose of 400 mg/day.
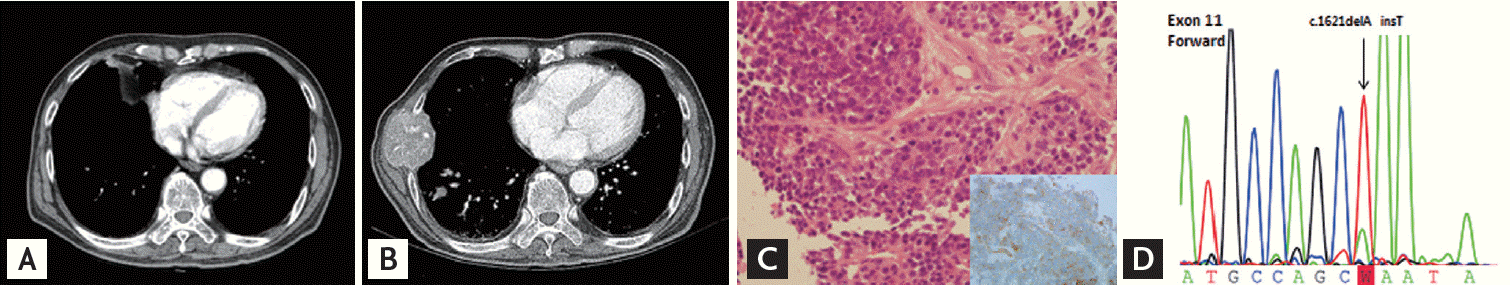
One month after imatinib treatment, he complained of jaundice, and laboratory findings showed direct hyperbilirubinemia. Chest and abdomen CT scans revealed that the stomach lesion was slightly decreased in size with necrosis (Fig. 1B). On the other hand, size and number of metastatic nodules of lung and liver were increased. Multiple new bone metastases were also found (Fig. 3B). To determine possible reasons for the different responses, we biopsied the metastatic right rib mass, which was confirmed as metastatic GIST with CD117 positivity. However, the KIT mutation was different from the primary site, with an exon 10 Met541Leu mutation (Fig. 3C and 3D). Histologically, the spindle tumor cells showed more round epithelioid cell features and increased mitotic figures than tumor cells of primary site. CD117 was weak but diffusely stained in the tumor cell membranes. However, immunochemistry of CD34, Dog-1, chromogranin, CD56a, CD99, S100, and HMB45 was negative. Despite imatinib dose escalation (800 mg/day) and palliative radiotherapy, the patient died after 3 weeks due to disease progression.
In this case, primary gastric GIST showed deletion and insertion at codons 557 and 558 of KIT exon 11, while the metastatic rib mass showed the M541L variant of KIT exon 10. After starting imatinib, the gastric lesion slightly decreased, but other metastatic lesions rapidly progressed, and the patient died within 2 months. Considering the very short time to progression, we postulate that the novel KIT exon 10 mutation is related to primary resistance and poor prognosis. For acquired resistance to tyrosine kinase inhibitor, secondary mutations have been found in more than 50% of tumors with primary KIT mutations, and intra- or intertumoral genetic heterogeneity has also been reported in GIST [3]. However, to the best of our knowledge, distinct genetic alteration between primary and metastatic tumors resulting in primary resistance has not been previously reported.
Mutations of KIT exon 10 have not been previously reported in GIST. Exon 10 of KIT is located between exons 9 and 11, which encode the extracellular and juxtamembrane domains, respectively. A previous study showed that a deletion at the intron 10-exon 11 boundary was found in 3.9% of exon 11 mutations of GIST [4]. The M541L mutation, as shown in our case, or the V530I mutation of exon 10 have been reported in patients with aggressive fibromatosis, but patents with these mutations showed good response to imatinib, in contrast to our case [5]. Further studies are needed to determine the clinical significance of the exon 10 mutation in GIST.
When multiple tumors show different responses to imatinib in metastatic GIST, distinct genetic mutations could be one reason for primary resistance. Genetic analyses of multiple metastatic sites should be performed to decide further treatment.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





