 |
 |
| Korean J Intern Med > Volume 32(3); 2017 > Article |
|
Abstract
Background/Aims
Approximately 30% of esophageal cancer (EC) patients cannot complete endoscopic ultrasonography (EUS) due to malignant stricture (EUS non-traversability). This study examines clinical implications of EUS non-traversability in patients with advanced locoregional squamous EC receiving preoperative chemoradiotherapy (CRT) followed by esophagectomy.
Methods
We retrieved data on 89 consecutive patients with advanced locoregional squamous EC (stage II or III). Relevant clinical and tumor-specific parameters were reviewed retrospectively. Significant factors affecting survival was determined by Cox regression analysis.
Results
EUS non-traversable EC was observed in 26 of 89 patients (29.2%). Median serum albumin level (3.6 g/dL vs. 3.9 g/dL, p = 0.028), tumor length (6.0 cm vs. 4.0 cm, p = 0.002), and percentage of clinical stage III disease (65.4% vs. 38.1%, p = 0.019) were significantly different between the patients with EUS non-traversable and traversable EC, respectively. Patients with EUS non-traversable EC demonstrated a significantly lower 5-year overall survival than patients with EUS traversable EC (30.8% vs. 49.3%, p = 0.023). In multivariate analysis, weight loss Ōēź 10% (p = 0.033), EUS non-traversability (p = 0.003), non-response to preoperative CRT (p = 0.002), and incompletion of esophagectomy (p = 0.002) were significant negative factors of survival.
Preoperative chemoradiotherapy (CRT) followed by esophagectomy is a well-known treatment modality for advanced locoregional esophageal cancer (EC) [1,2]. This multimodality therapy has demonstrated survival benefit and has become a standard of care. Endoscopic ultrasonography (EUS) plays a central role in planning a stage-specific treatment for patients with EC [3]. The great advantage of EUS is its ability to determine the depth of tumor invasion and regional node metastasis [4,5].
However, 25% to 32% of patients with advanced EC present with severe stenosis and a radial echoendoscope is unable to pass through the lesion (EUS non-traversability) [6-8]. Although dilatation of EC stricture for EUS staging has been applied in some cases, the procedure carries a potential risk of luminal perforation [9]. Thus tumor stage and treatment plans in such EUS non-traversable EC is determined mostly by conventional imaging work-ups.
Non-traversable esophageal stricture was reported to be a factor related to prognosis in EC patients receiving definitive CRT [10]. However, studies on the clinical implications of EUS non-traversability in patients with advanced locoregional squamous EC receiving preoperative CRT and esophagectomy has been limited, and we therefore sought to determine its significance in this specific group of patients.
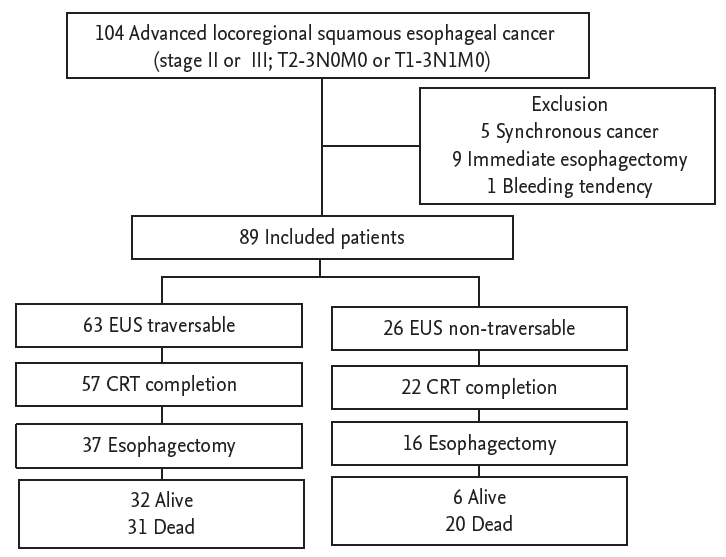
Between June 2005 and December 2007, a total of 310 patients were diagnosed with squamous EC in Asan Medical Center. A retrospective review of the consecutive patients identified 104 patients with advanced locoregional EC (stage II or III; T2ŌĆō3N0M0 or T1ŌĆō3N1M0). Among those, 15 patients were excluded from the analysis: five patients had synchronous second primary cancer, nine received immediate esophagectomy, and 1 did not undergo EUS due to bleeding tendency. Thus, a total of 89 patients were included in this study (Fig. 1). All patients were treated with preoperative CRT, including 53 patients who were treated with preoperative CRT followed by esophagectomy. At the last follow-up date of the patients, 32 of 63 patients in EUS traversable group and 6 of 26 patients in EUS non-traversable group were alive.
Clinical and tumor-specific data were routinely collected. Underlying nutritional status was evaluated in each patient, including serum albumin level and percent weight loss in the preceding 6 months. Dysphagia to semisolid or liquid food was recorded. Self-expanding metal stents were inserted for palliation of dysphagia in patients with severe EC stenosis. Performance status was assessed according to Eastern Cooperative Oncology Group (ECOG) score.
All patients underwent esophagogastroscopy with biopsy, EUS, esophagography, chest-abdomen computed tomography (CT) with contrast, and 18F-fluorodeoxyglucose positron emission tomography CT (PET-CT). Bronchoscopy was performed for EC located at or above the carina. Chromoendoscopy with lugol-staining was carried out to measure the length of each tumor prior to EUS. In EUS non-traversable EC, clinical stage was determined based on conventional work-ups including esophagography, chest-abdomen CT, and PET-CT. Tumor stage was determined using the TNM staging system [11]. This study was approved by the Institutional Review Board of the Asan Medical Center.
EUS was performed using a radial-scanning echoendoscope (GF-UMQ240 or GF-UM2000: distal end outer diameter, 12.7 mm and 10.5 mm, respectively; Olympus, Tokyo, Japan) with 7.5- and 12-MHz transducer. T stage using EUS was determined by directly observing the depth of hypoechoic expansion of a tumor through the five layers of the esophageal wall. T1 tumors involve the mucosa and submucosa (layers 1 to 3), and T2 tumors invade into the muscularis propria (layers 1 to 4). T3 tumors penetrate beyond the smooth outer border of the muscularis propria, indicating invasion into the adventitia. T4 tumors involve the adjacent organs, such as the major vessels, trachea, pericardium, and pleura, via contiguous invasion. Lymph nodes were considered metastatic (N1) if they met Ōēź 3 of the following four criteria: hypoechoic, size > 5 mm in short diameter, round shape, and well-demarcated border [12,13].
Patients with advanced locoregional squamous EC were treated with preoperative CRT followed by esophagectomy. Briefly, preoperative CRT consisted of cisplatin plus fluoropyrimidine-based chemotherapy with concurrent radiotherapy (46 Gy/23 fractions) over 4 weeks. Clinical response to preoperative CRT was evaluated 4 weeks after the end of radiotherapy using esophagogastroscopy with biopsy, esophagography, chest-abdomen CT, and PET-CT. Subsequent esophagectomy was performed with a transhiatal, abdominal-right thoracic (Ivor-Lewis) or right thoracic-abdominal-cervical (McKeown) approach. All patients were followed up with physical examination, esophagogastroscopy, and chest-abdomen CT every 3 months for 2 years, then every 6 months for the next 3 years.
Baseline variables are presented as number (percentage) and median (interquartile range [IQR]). Continuous variables were compared using the Student t test, and categorical variables were compared using the chisquare test or Fisher exact test. Survival outcomes were measured from the date of EC diagnosis to the date of death from any cause or the last visit. Overall survival was calculated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate analyses with Cox proportional hazards modeling were performed to determine the significant factors that affect patient survival. All p values were two-sided, and a p < 0.05 was considered significant. All statistical analyses were performed using SPSS version 21.0 (IBM Co., Armonk, NY, USA).
The median age of the study patients was 62 years (IQR, 57.5 to 66.0) and 86 patients were male (Table 1). At diagnosis, 56 patients (62.9%) presented with dysphagia and 13 (14.6%) had developed Ōēź 10% weight loss in the previous 6 months. A radial echoendoscope could not pass through EC in 26 of 89 patients (29.2%). These patients with EUS non-traversable EC showed a higher rate of dysphagia (88.5% vs. 52.4%, p = 0.001) and need for insertion of self-expanding esophageal stent (30.8% vs. 1.6%, p < 0.001) than patients with EUS traversable EC. The median serum albumin level (3.6 g/dL vs. 3.9 g/dL, p = 0.028), tumor length (6.0 cm vs. 4.0 cm, p = 0.002), and percentage of clinical stage III disease (65.4% vs. 38.1%, p = 0.019) were significantly different between the patients with EUS non-traversable and traversable EC. Weight loss and ECOG performance status did not differ between the groups.
Of the 89 patients, 79 (88.8%) completed the planned CRT schedule: 22 of 26 patients (84.6%) with EUS non-traversable EC and 57 of 63 patients (90.5%) with EUS traversable EC (p = 0.426) (Fig. 1). Complete or partial response to preoperative CRT was observed in 70 patients, and the rates did not differ between EUS non-traversable and traversable EC patients (76.9% vs. 79.4%, respectively; p = 0.798) (Table 1). In total, 53 patients (59.6%) underwent esophagectomy: 16 (61.5%) EUS non-traversable and 37 (58.7%) EUS traversable EC patients (p = 0.806). Ivor-Lewis operation was performed in 38 cases (71.7%), McKeown operation in 14 cases (26.4%), and esophagocolojejunostomy in one patient (1.9%). The reasons for 36 patients who did not undergo esophagectomy were patientsŌĆÖ refusal with symptom relief in 17 (47.2%), disease progression in 14 (38.9%), inoperable condition in three (8.3%), and suspended surgery due to unresectable intraoperative finding in two patients (5.6%). Two patients died of postoperative severe infection in the EUS traversable EC group.
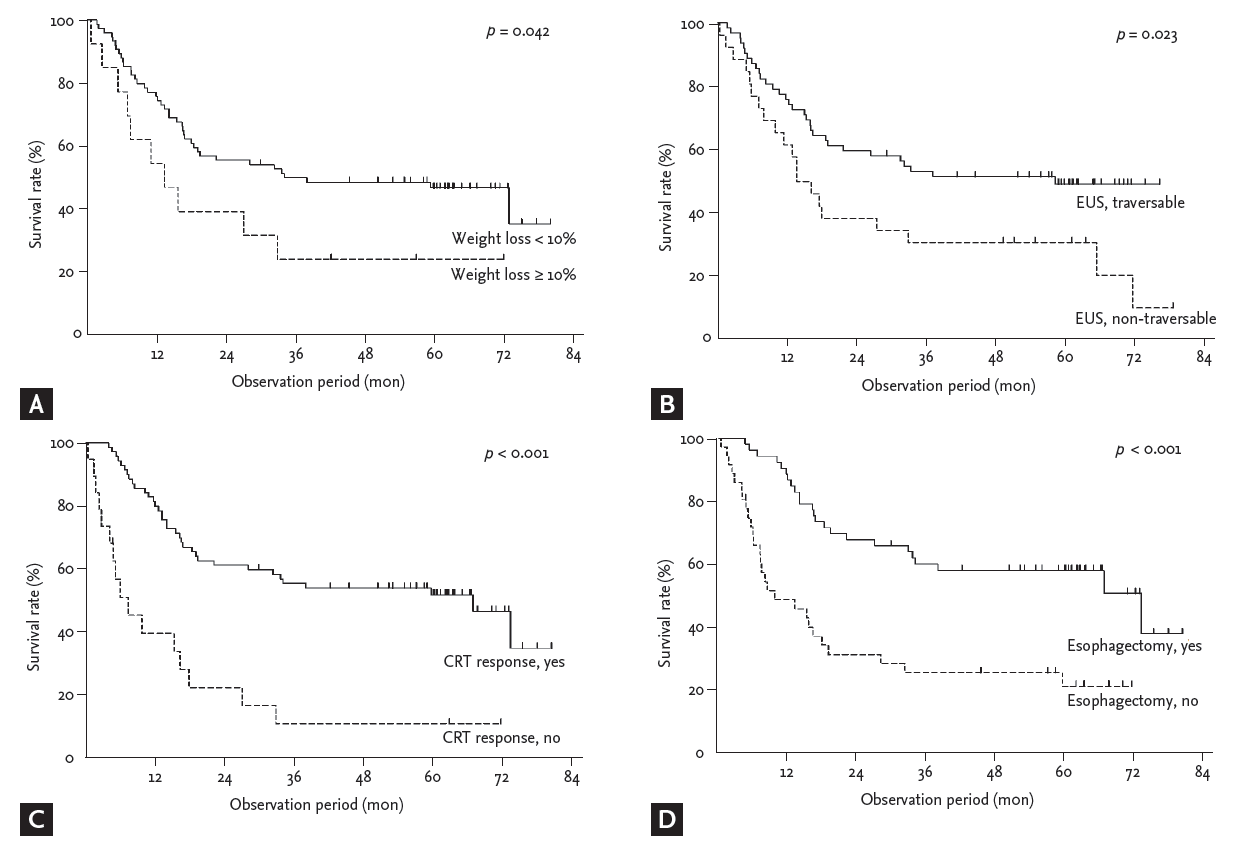
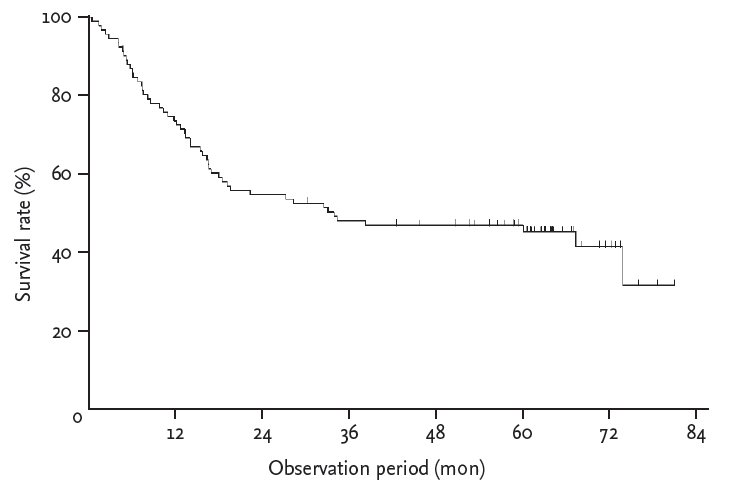
After a median follow-up of 29.9 months (IQR, 10.0 to 62.0), the median overall survival of all patients was 32.8 months (95% confidence interval, 0 to 68.2) and the 5-year survival rate was 43.8% (Fig. 2). Patients with EUS non-traversable EC showed a significantly lower 5-year overall survival than patients with EUS traversable EC (30.8% vs. 49.3%, p = 0.023) (Fig. 3). Univariate analysis identified that weight loss Ōēź 10% (p = 0.047), serum albumin level < 3.8 g/dL (p = 0.035), and EUS non-traversability (p = 0.025) were significant negative factors of survival (Table 2). Non-response to preoperative CRT (p < 0.001) and incompletion of planned esophagectomy (p < 0.001) were also identified as negative prognostic factors by univariate analysis. Weight loss Ōēź 10% (p = 0.033), EUS non-traversability (p = 0.003), non-response to preoperative CRT (p = 0.002), and incompletion of esophagectomy (p = 0.002) remained significant negative factors of survival in multivariate analysis (Table 2, Fig. 3).
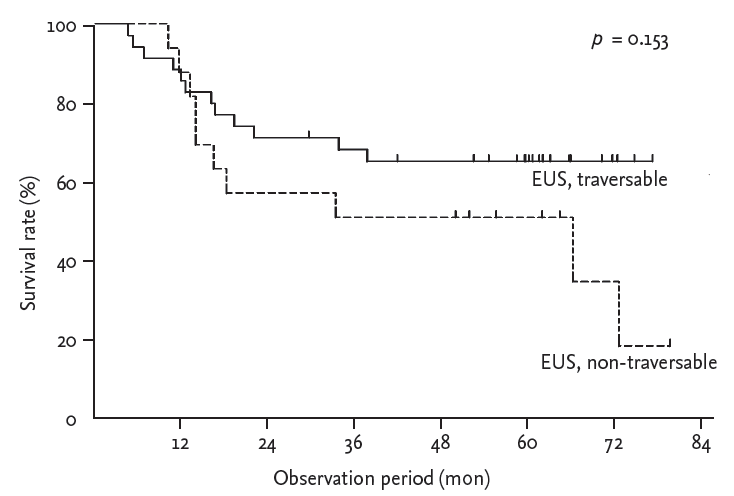
Five-year survival rate was 50.0% when 16 patients with EUS non-traversable EC achieved a clinical response to preoperative CRT and also underwent esophagectomy (vs. 64.5% of 34 patients with EUS traversable EC; p = 0.153) (Fig. 4).
We show from our current findings that EUS non-traversability has significant negative prognostic implications in patients with advanced locoregional squamous EC receiving preoperative CRT followed by esophagectomy. Patients with EUS non-traversable EC demonstrated a lower 5-year overall survival than patients with EUS traversable EC.
The poor prognosis of EUS non-traversable EC patients in the current study can be explained firstly by more advanced locoregional stage and a larger tumor burden. EUS non-traversable EC stricture was noted in 29.2% of our patients. These patients presented with dysphagia, a lower serum albumin level and a larger tumor length. In addition, a significant portion of those had clinical stage III disease (65.4% vs. 38.1%).
The association between EUS non-traversability and advanced EC stage has previously been reported. When 79 patients with EC were staged preoperatively using EUS and compared with the pathologic stage of the esophagectomy specimen, 91% of the patients with malignant stricture had stage III or IV disease [8]. In another study conducted with 167 EC patients undergoing immediate surgery also reported that 88% of EUS non-traversable patients had T3 or T4 disease [14]. However, in our clinical setting of patients with stage II or III disease undergoing multimodality therapy, the impact of tumor stage on the prognosis may have been mitigated by response to preoperative CRT.
Dysphagia was more frequently found in EUS non-traversable EC patients in the current study. We speculate that EUS non-traversability may have similar clinical implications to dysphagia, which is a well-known prognostic factor of EC [15-17]. However, dysphagia is a subjective symptom which inherently bears limited reliability. In addition, other etiologies including esophagitis and gastroesophgeal reflux disease can mimic similar symptom in the absence of esophageal obstruction [18,19]. Therefore, non-passage of EUS scope with a fixed outer diameter may serve as a more objective and simple indicator for prognosis in lieu of dysphagia. Esophageal stricture with luminal diameter of less than 13 mm has previously been regarded crucial to the presence of dysphagia [20,21].
In addition, the prognosis of our patients with EUS non-traversable EC may have been further worsened by the limited EUS assessment of EC stage. EUS is a standard locoregional staging modality for EC, demonstrating high T (80% to 90%) and N staging accuracy (70% to 80%) that is clearly superior to CT and magnetic resonance imaging [3,4]. However, the accuracy of EUS decreases significantly when an echoendoscope cannot pass through EC. Staging accuracy of EUS is reportedly 46% in EUS non-traversable EC (vs. 92% in EUS traversable EC) and correct preoperative T stage was obtained using EUS only in 30.8% of patients with high-grade EC stenosis (vs. 81% of patients with less severe EC stenosis) [6,22]. A previous study reported that 9.9% of patients with distant metastatic nodes were found on EUS after EC stenosis dilation [23]. Another study on the effects of EUS after dilation of EC stenosis reported that EUS detected additional cases of advanced diseases in 19% of patients, including celiac node involvement and T4 disease [7]. However, dilation of EC stenosis has not been performed routinely because of a considerable risk of tumor perforation [6,8,9,24], and the EC stage in such cases was determined on the basis of conventional staging work-ups. Although the pathologic stage was not evaluable in our patients due to the effect of preoperative CRT, these evidences suggest that preoperative CRT followed by esophagectomy for some of our patients with non-traversable EC may have been a stage-inappropriate treatment and thus adversely affected survival outcome. Development of newer echoendoscope with smaller diameter, which still retain its staging accuracy, seems to be beneficial to the patients with stenotic EC.
Preoperative CRT and esophagectomy are two main constituents for the treatment of advanced locoregional squamous EC [1,2,16,17,25-27]. We previously reported that clinical response to preoperative CRT was a significant prognostic factor of survival in patients with advanced locoregional EC, and survival was better in patients who subsequently underwent esophagectomy [17]. In our current study, the rate of clinical response to preoperative CRT was similar between EUS non-traversable and traversable EC patients (76.9% vs. 79.4%, respectively). Patients with EUS non-traversable EC also showed comparable compliance to multimodality therapy: 84.6% of these patients finished the planned preoperative CRT and 61.5% underwent esophagectomy. Palliation of dysphagia and nutritional support are important parts of EC management, and can be achieved by insertion of esophageal stent [15,28]. In the present study, 30.8% of EUS non-traversable EC patients received self-expanding esophageal stent before treatment, which led to satisfactory compliance and high rate of response to CRT.
In EUS non-traversable and traversable EC patients, the favorable 5-year overall survival was observed in patients who achieved clinical response to preoperative CRT and also underwent planned esophagectomy (50.0% and 64.5%, respectively). This finding suggests that esophagectomy should be considered for the cure of EC even in stenotic patients who refuse surgery after symptom relief with preoperative CRT.
The 5-year overall survival (43.8%) in our patients is consistent with previous studies conducted in locally advanced EC patients. A recent multicenter trial reported median overall survival of 49.4 months with 5-year overall survival of 47% for patients in CRT-surgery group [2]. In another trial comparing the effect of adding preoperative CRT to surgery alone, patients with multimodality treatment group showed median overall survival of 4.48 years with 5-year overall survival of 39% [29]. In contrast, survival was generally lower when only CRT was applied to patients in similar clinical stage. The median overall survival was reportedly 16.2 months when patients in whom the majority was in stage III underwent definitive CRT [15]. Another review of 143 EC patients reported 5-year overall survival of 19.8% with median overall survival of 22.1 months when patients received definitive CRT [10].
The key findings of our study are the negative prognostic implications of EUS non-traversability in advanced locoregional EC patients receiving preoperative CRT and esophagectomy. This result may be ascribed to more advanced clinical stage, a larger tumor burden, and limitation of accurate EUS tumor staging. Our present results also suggest that treatment should be continued in patients with EUS non-traversable EC, given this groupŌĆÖs satisfactory compliance with multimodality therapy and the favorable survival of non-traversable patients who achieved preoperative CRT response and also finished the planned esophagectomy.
Our study has some limitations inherent to its retrospective design and use of observational data collected in a single tertiary center. In addition, factors known to be important to survival, such as the details of additional chemotherapy and/or radiation therapy, could not be estimated in this study.
1. Endoscopic ultrasonography (EUS) non-traversability has negative prognostic implications in advanced locoregional esophageal cancer (EC) patients receiving preoperative chemoradiotherapy (CRT) and esophagectomy.
2. The negative prognostic implications may be ascribed to more advanced clinical stage, a larger tumor burden, and limitation of accurate EUS tumor staging.
3. The survival of non-traversable EC patients was favorable when they achieved preoperative CRT response and also finished the planned esophagectomy.
Figure┬Ā3.
Kaplan-Meier curves for overall survival segregated according to (A) weight loss, (B) endoscopic ultrasonography (EUS) traversability, (C) clinical response to preoperative chemoradiotherapy (CRT), and (D) esophagectomy (p values in log-rank test).
Figure┬Ā4.
Kaplan-Meier curves for overall survival. Fiveyear survival rate was 50.0% when patients with endoscopic ultrasonography (EUS) non-traversable esophageal cancer (EC) achieved a clinical response to preoperative chemoradiotherapy and also underwent esophagectomy (vs. 64.5% of patients with EUS traversable EC) (p values in log-rank test).
Table┬Ā1.
Clinical features of patients with EUS traversable and non-traversable EC
| Variable | Total (n = 89) | EUS traversable EC (n = 63) | EUS non-traversable EC (n = 26) | p value |
|---|---|---|---|---|
| Age, yr | 62.0 (57.5ŌĆō66.0) | 62.0 (57.0ŌĆō66.0) | 61.0 (57.5ŌĆō66.3) | 0.962 |
| Male sex | 86 (96.6) | 61 (96.8) | 25 (96.2) | 1.000 |
| Smoking | 69 (77.5) | 48 (76.2) | 21 (80.8) | 0.638 |
| Alcohol | 77 (86.5) | 57 (90.5) | 20 (76.9) | 0.101 |
| Dysphagia | 56 (62.9) | 33 (52.4) | 23 (88.5) | 0.001 |
| Weight lossa | 13 (14.6) | 9 (14.3) | 4 (15.4) | 1.000 |
| Albumin, g/dL | 3.8 (3.5ŌĆō4.1) | 3.9 (3.6ŌĆō4.1) | 3.6 (3.4ŌĆō4.0) | 0.028 |
| ECOG-PS | 0.106 | |||
| ŌĆā0 | 24 (27.0) | 21 (33.3) | 3 (11.5) | |
| ŌĆā1 | 60 (67.4) | 39 (61.9) | 21 (80.8) | |
| ŌĆāŌēź 2 | 5 (5.6) | 3 (4.8) | 2 (7.7) | |
| Tumor length, cm | 5 (3ŌĆō7) | 4 (2ŌĆō6) | 6 (4ŌĆō7) | 0.002 |
| Tumor location | ||||
| ŌĆāUpper third | 6 (6.7) | 5 (7.9) | 1 (3.8) | 0.143 |
| ŌĆāMiddle third | 25 (28.1) | 11 (17.5) | 14 (53.8) | |
| ŌĆāLower third | 58 (65.2) | 47 (74.6) | 11 (42.3) | |
| Clinical stageb | 0.019 | |||
| ŌĆāII | 48 (53.9) | 39 (61.9) | 9 (34.6) | |
| ŌĆāIII | 41 (46.1) | 24 (38.1) | 17 (65.4) | |
| Preoperative CRT response | 0.037 | |||
| ŌĆāComplete response | 40 (44.9) | 34 (54.0) | 6 (23.1) | |
| ŌĆāPartial response | 30 (33.7) | 16 (25.4) | 14 (53.8) | |
| ŌĆāStable disease | 4 (4.5) | 3 (4.8) | 1 (3.8) | |
| ŌĆāProgressive disease | 15 (16.9) | 10 (15.9) | 5 (19.2) | |
| Stent insertion | 9 (10.1) | 1 (1.6) | 8 (30.8) | < 0.001 |
| Esophagectomy | 53 (59.6) | 37 (58.7) | 16 (61.5) | 0.806 |
Table┬Ā2.
Significant factors of survival in univariate and multivariate analyses
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| p value | p value | HR | 95% CI | |
| Age, yr | 0.994 | - | ||
| ŌĆāŌēź 62 (n = 44) | ||||
| ŌĆā< 62 (n = 45) | ||||
| Sex | 0.821 | - | ||
| ŌĆāMale (n = 86) | ||||
| ŌĆāFemale (n = 3) | ||||
| Weight loss, %a | 0.047 | 0.033 | ||
| ŌĆā< 10 (n = 76) | 1 | |||
| ŌĆāŌēź 10 (n = 13) | 2.17 | 1.06ŌĆō4.44 | ||
| Serum albumin level | 0.035 | 0.065 | ||
| ŌĆāŌēź 3.8 (n = 50) | 1 | |||
| ŌĆā< 3.8 (n = 39) | 1.71 | 0.97ŌĆō3.04 | ||
| ECOG performance status | 0.554 | - | ||
| ŌĆā0, 1 (n = 84) | ||||
| ŌĆāŌēź 2 (n = 5) | ||||
| EUS traversability | 0.025 | 0.003 | ||
| ŌĆāYes (n = 63) | 1 | |||
| ŌĆāNo (n = 26) | 2.47 | 1.37ŌĆō4.46 | ||
| Tumor length, cm | 0.069 | - | ||
| ŌĆā< 5 (n = 39) | ||||
| ŌĆāŌēź 5 (n = 50) | ||||
| Tumor location | 0.493 | - | ||
| ŌĆāUpper/middle (n = 31) | ||||
| ŌĆāLower (n = 58) | ||||
| Clinical stage | 0.079 | - | ||
| ŌĆāII (n = 48) | ||||
| ŌĆāIII (n = 41) | ||||
| Clinical response to preoperative CRTb | < 0.001 | 0.002 | ||
| ŌĆāYes (n = 70) | 1 | |||
| ŌĆāNo (n = 19) | 2.83 | 1.47ŌĆō5.47 | ||
| Esophagectomy | < 0.001 | 0.002 | ||
| ŌĆāYes (n = 53) | 1 | |||
| ŌĆāNo (n = 36) | 2.64 | 1.41ŌĆō4.92 | ||
REFERENCES
1. Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol 2007;25:4110ŌĆō4117.


2. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074ŌĆō2084.


3. Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol 2005;23:4483ŌĆō4489.


4. Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001;49:534ŌĆō539.



5. Pfau PR, Perlman SB, Stanko P, et al. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc 2007;65:377ŌĆō384.


6. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535ŌĆō539.


7. Wallace MB, Hawes RH, Sahai AV, Van Velse A, Hoffman BJ. Dilation of malignant esophageal stenosis to allow EUS guided fine-needle aspiration: safety and effect on patient management. Gastrointest Endosc 2000;51:309ŌĆō313.


8. Van Dam J, Rice TW, Catalano MF, Kirby T, Sivak MV Jr. High-grade malignant stricture is predictive of esophageal tumor stage: risks of endosonographic evaluation. Cancer 1993;71:2910ŌĆō2917.


9. Jethwa P, Lala A, Powell J, et al. A regional audit of iatrogenic perforation of tumours of the oesophagus and cardia. Aliment Pharmacol Ther 2005;21:479ŌĆō484.


10. Clavier JB, Antoni D, Atlani D, et al. Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis Esophagus 2014;27:560ŌĆō567.


11. Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer, 2002.
12. Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc 1994;40:442ŌĆō446.


13. Vickers J, Alderson D. Oesophageal cancer staging using endoscopic ultrasonography. Br J Surg 1998;85:994ŌĆō998.


14. Dittler HJ, Siewert JR. Role of endoscopic ultrasonography in esophageal carcinoma. Endoscopy 1993;25:156ŌĆō161.


15. Di Fiore F, Lecleire S, Pop D, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol 2007;102:2557ŌĆō2563.


17. Kim MK, Kim SB, Ahn JH, et al. Treatment outcome and recursive partitioning analysis-based prognostic factors in patients with esophageal squamous cell carcinoma receiving preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys 2008;71:725ŌĆō734.


18. Bloem BR, Lagaay AM, van Beek W, Haan J, Roos RA, Wintzen AR. Prevalence of subjective dysphagia in community residents aged over 87. BMJ 1990;300:721ŌĆō722.



19. Triadafilopoulos G. Nonobstructive dysphagia in reflux esophagitis. Am J Gastroenterol 1989;84:614ŌĆō618.

20. Schatzki R. The lower esophageal ring: long term follow-up of symptomatic and asymptomatic rings. Am J Roentgenol Radium Ther Nucl Med 1963;90:805ŌĆō810.

21. Earlam R, Cunha-Melo JR. Benign oesophageal strictures: historical and technical aspects of dilatation. Br J Surg 1981;68:829ŌĆō836.


22. Hordijk ML, Zander H, van Blankenstein M, Tilanus HW. Influence of tumor stenosis on the accuracy of endosonography in preoperative T staging of esophageal cancer. Endoscopy 1993;25:171ŌĆō175.


23. Pfau PR, Ginsberg GG, Lew RJ, Faigel DO, Smith DB, Kochman ML. Esophageal dilation for endosonographic evaluation of malignant esophageal strictures is safe and effective. Am J Gastroenterol 2000;95:2813ŌĆō2815.


25. Lee JL, Park SI, Kim SB, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol 2004;15:947ŌĆō954.


26. Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310ŌĆō2317.


27. Kim CM, Hong WS, Lee JO, et al. A retrospective study on radiotherapy and radiochemotherapy in esophageal cancer. Korean J Intern Med 1988;3:58ŌĆō63.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



