To the Editor,
Wernicke encephalopathy (WE) is an acute neuropsychiatric syndrome caused by thiamine deficiency and is associated with significant morbidity and mortality. WE has an acute onset and may present as the classic triad of encephalopathy, ocular changes, and gait ataxia. However, the combination of all three is only observed in one-third of patients with WE [1]. Although WE usually results from chronic alcohol dependency, nonalcoholic causes are reported in 20% to 50% of patients. WE uncommonly develops in patients with cancer [2,3]. There are few case reports of WE in patients with malignant lymphoma [3]. We herein present an uncommon case of WE associated with non-Hodgkin lymphoma; notably, the WE developed before the diagnosis of the cancer. We also present a review of the medical literature on previously reported cases of WE associated with lymphoma.
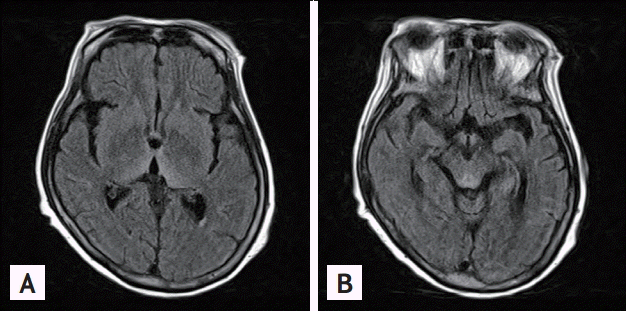
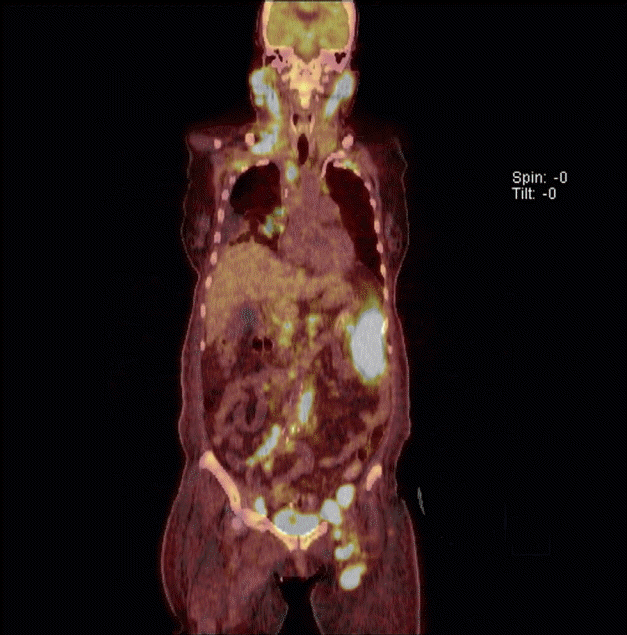
A 64-year-old female was brought to the Emergency Department with a 10-hour history of unexplained coma and a 10-day history of ataxia. She had lost 18 kg of body weight in the previous 2 months. She had no history of alcohol use. Physical examination revealed a fever (body temperature of 41┬║C) and dehydration, but was otherwise unremarkable. Neurologic examination was negative for neck stiffness, the Kernig sign, and the Brudzinski sign; however, upbeating nystagmus was present. Cerebellar function tests were limited because of the patientŌĆÖs mental status. The initial A Glasgow Coma Scale (GCS) score was 4 (E1V1M2). The patientŌĆÖs laboratory findings were as follows: white blood cell count, 8.03 ├Ś 109/L with 40% neutrophils and 50% lymphocyte; hemoglobin, 12.5 g/dL; platelets, 34 ├Ś 109/L; total protein, 7.9 g/dL; albumin, 3.8 g/dL; blood urea nitrogen, 42.7 mg/dL; creatinine, 1.98 mg/dL; aspartate aminotransferase, 32 IU/L; alanine transaminase, 11 IU/L; sodium, 145 mEq/L; potassium, 4.0 mEq/L; lactate dehydrogenase, 1,503 U/L; calcium, 8.8 mg/dL; phosphorus, 4.2 mg/dL; magnesium, 3.0 mg/dL; osmolality, 342 mOsm/kg; thyroid-stimulating hormone, 0.18 mIU/L; free thyroxine, 1.02 ng/dL; ferritin, 1,650 ng/mL; ammonia, 73 ┬Ąg/dL; prothrombin time, 52.2% (international normalized ratio, 1.35); and activated partial thromboplastin time, 14.8 seconds. The C-reactive protein and erythrocyte sedimentation rate were 0.88 mg/dL and 2 mm/hr, respectively, and the blood culture was negative for bacteria and fungi. Initial electrocardiogram and chest X-ray study were normal. Arterial blood gas analysis showed a pH of 7.549, pCO2 of 17.7 mmHg, pO2 of 88.1 mmHg, HCO3 of 15.1 mmol/L, and saturation of 96.8%. A lumbar puncture demonstrated clear color, lactate dehydrogenase 64 IU/L, glucose 59 mg/dL (reference range, 40 to 70), protein 72 mg/dL (reference range, 15 to 45), and no pleocytosis. Cerebrospinal fluid cytology reveled negative finding for malignancy. There was no evidence of central nervous system infection. Brain magnetic resonance imaging (MRI) revealed characteristic typical findings of WE, namely symmetrical high signal intensity in the thalamic and periaqueductal area on T2-weighted images with no other abnormalities in the brain parenchyma or extra-axial space (Fig. 1). The patient was treated with 100 mg of intravenous thiamine, and her neurologic symptoms improved (GCS, E2V2M3). To evaluate her febrile state, she underwent abdominal computed tomography, which revealed multiple enlarged, homogeneously enhancing lymph nodes in the para-aortic, bilateral common iliac, femoral, and inguinal areas. Positron emission tomography demonstrated high uptake in the spleen, bone marrow, and multiple lymph nodes in cervical, thoracic and abdominal areas (Fig. 2). Biopsy of the inguinal lymph nodes demonstrated anaplastic large T cells that were positive for surface CD3, CD30, and Bcl-2, but negative for ALK-1. The Ki-67 proliferation index was 80%. In situ hybridization was strongly positive for Epstein-Barr virus-encoded small RNAs. The patientŌĆÖs bone marrow biopsy showed normal cellularity and involvement of lymphoma. Her Ann Arbor stage was IVB, and her international prognostic index risk group was high. She received a cycle of chemotherapy with the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisolone), and her nystagmus improved, although her mental status was similar to that of the last examination. However, during chemotherapy, she died of sepsis secondary to treatment-related pneumonia.
WE is an acute neurologic condition associated with thiamine deficiency. It is characterized by mental status changes, oculomotor dysfunction, and motor problems, such as gait incoordination and ataxia [2,3]. WE is associated with significant morbidity and mortality and, if left untreated, coma and death. While WE is primarily a clinical diagnosis, laboratory studies and neuroimaging may be helpful. Measurements of the blood thiamine concentration or the red blood cell transketolase activity could be a presumptive diagnostic tool. However, these laboratory measurements are limited by low specificity and technical difficulty [2]. MRI is currently considered to be the most valuable method with which to confirm a diagnosis of WE. MRI findings reveal high T2 signal intensities, bilaterally symmetrical in the paraventricular regions of the thalamus, hypothalamus, mammillary bodies, periaqueductal region, floor of the fourth ventricle, and midline cerebellum [2,4]. Rapid recovery after the administration of intravenous thiamine is in itself diagnostic of thiamine deficiency [2]. Our patient had mental status changes and ocular abnormalities at initial presentation. The MRI scans in the present case showed typical findings of WE, although measurement the thiamine level was not available. We administered high doses of thiamine, and her neurologic status improved.
WE is usually associated with chronic alcohol consumption. Nonalcoholic causes are present in 20% to 50% of cases and include a staple diet of polished rice, parenteral nutrition, eating disorders, cancer and its treatment, severe infection, and gastrointestinal surgery [2,3]. Although uncommonly reported, WE has also been described in patients within operable gastric carcinoma, non-Hodgkin lymphoma, myelomonocytic leukemia, large B-cell lymphoma, myeloid leukemia, allogenic bone marrow transplantation, and even early stage cancer [2].
The relationship between cancer and thiamine has not been clearly determined [3]. Thiamine deficiency could be associated with the presence of the cancer cells themselves. In addition, poor dietary intake related to lack of appetite and recurrent nausea from chemotherapy or radiotherapy has been suggested as possible causes for cancer-associated WE [2]. In our patient, who developed WE before chemotherapy, both the active consumption of thiamine by lymphoma cells and malnutrition associated with extensive lymphoma involvement may have been major contributing factors to the development of WE. Several genetic factors related to thiamine and cancer could also be involved, such as the solute carrier transporter gene (SCL19), transketolase, transcription factor p53, the poly (ADP-ribose) polymerase-1 gene (PARP1), and the reduced form of nicotinamide adenine dinucleotide phosphate [5]. Thiamine deficiency in patients with lymphoma has not been thoroughly studied. Almost all reported patients developed WE after chemotherapy, although our patient developed WE before the diagnosis of lymphoma. Therefore, parenteral thiamine may be prophylactically administered to any patients receiving high-dose chemotherapy and exhibiting the possibility of WE. WE is a medical emergency; when the disorder is suspected, thiamine should be given immediately before or concomitantly with intravenous administration of glucose [2]. Any therapeutic delay may result in permanent neurological damage or death. Clinicians must remain aware of the possibility of WE when any patient presents with unexplained mental changes such as coma or a stuporous state, particularly if there is a known causal factor associated with WE.
We have herein reported an uncommon case of WE in a patient who was admitted with the chief neurologic symptoms of a comatose state and nystagmus. Evaluation of her fever subsequently led to the diagnosis of non-Hodgkin lymphoma. Although very rare, the possibility of WE should be considered when any patient with lymphoma develops unexplained neurologic symptoms such as mental status changes. Immediate parenteral administration of high-dose thiamine even before a definitive diagnosis may be recommended because WE is reversible; however, if untreated, it is complicated by coma and death. Further investigations on the roles of thiamine deficiency and prophylactic parenteral thiamine in patients with cancer are needed.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





