 |
 |
| Korean J Intern Med > Volume 31(1); 2016 > Article |
|
Abstract
Background/Aims:
The CHADS2 score, used to predict the risk of ischemic stroke in atrial fibrillation (AF) patients, has been reported recently to predict ischemic stroke in patients with coronary heart disease, regardless of the presence of AF. However, little data are available regarding the relationship between the CHADS2 score and cardiovascular outcomes.
Methods:
This was a retrospective study on 104 patients admitted for acute coronary syndrome (ACS) who underwent coronary angiography, carotid ultrasound, and transthoracic echocardiography.
Results:
The mean age of the subjects was 60.1 ┬▒ 12.6 years. The CHADS2 score was as follows: 0 in 46 patients (44.2%), 1 in 31 (29.8%), 2 in 18 (17.3%), and Ōēź 3 in 9 patients (8.7%). The left atrial volume index (LAVi) showed a positive correlation with the CHADS2 score (20.8 ┬▒ 5.9 for 0; 23.2 ┬▒ 6.7 for 1; 26.6 ┬▒ 10.8 for 2; and 30.3 ┬▒ 8.3 mL/m2 for Ōēź3; p = 0.001). The average carotid total plaque area was significantly increased with CHADS2 scores Ōēź 2 (4.97 ┬▒ 7.17 mm2 vs. 15.52 ┬▒ 14.61 mm2; p = 0.002). Eight patients experienced cardiovascular or cerebrovascular (CCV) events during a mean evaluation period of 662 days. A CHADS2 score Ōēź 3 was related to an increase in the risk of CCV events (hazard ratio, 14.31; 95% confidence interval, 3.53 to 58.06). Furthermore, LAVi and the severity of coronary artery obstructive disease were also associated with an increased risk of CCV events.
The CHADS2 score (scored as 1 point each for presence of congestive heart failure, hypertension, diabetes, or age Ōēź 75 years; and 2 points each for prior stroke or transient ischemic attack) has been used to estimate the risk of ischemic stroke in patients with atrial fibrillation (AF) [1]. Recent data have demonstrated that this score can predict ischemic stroke even in patients with stable angina or acute coronary syndrome (ACS), irrespective of the presence of AF [2-4]. The CHADS2 score was also introduced as a predictor of stroke severity, functional outcomes in AF patients, and short-term functional outcomes in stroke patients with a medical history of coronary heart disease (CHD) [4,5]. However, whether the CHADS2 score can predict cardiovascular events in patients of ACS remains unknown.
The predictive value of the left atrial volume index (LAVi), based on corrected left atrial volume (LAV) divided by the body surface area, has been established for cardiovascular events [6-8]. LAV reflects the cardiac burden resulting from the chronic elevation of left ventricular filling pressure and diastolic dysfunction [9]. Elevated LAVi has been reported as a poor prognostic factor for patients with cardiac diseases such as myocardial infarction and heart failure [6,10,11]. Some studies have reported that increased LAV or LAVi can predict poor cardiovascular outcomes even in the general population [12,13].
Another factor considered to have a possible prognostic value for cardiovascular outcomes is atherosclerosis of the carotid artery, i.e., intima-media thickness (IMT) and carotid plaque area [14]. The carotid total plaque burden may predict outcomes of stroke and myocardial infarction [15,16]. However, unlike information for LAVi, data regarding the relationship between the carotid plaque burden and cardiovascular outcomes remain insufficient [17].
We hypothesized that the CHADS2 score can predict cardiovascular and cerebrovascular (CCV) events in ACS patients with documented coronary artery disease without AF. We used two main parameters for estimating the outcomes: the direct value from the actual CCV event and the indirect value reflecting adverse CCV outcomes, i.e., LAVi, which is already known as an adverse predictor of cardiovascular events. In addition, we also assessed the carotid plaque area and IMT. Thus, we investigated the correlation between the CHADS2 score and carotid total plaque area with LAVi for the evaluation of cardiovascular outcomes.
This retrospective cohort study included patients from a single medical center (Ewha Womans University Mokdong Hospital, Korea). The medical records of patients hospitalized for ACS who underwent coronary angiography between July 2008 and August 2013 were reviewed. The inclusion criteria were as follows: (1) patients admitted for ACS who underwent coronary angiography that revealed coronary artery obstructive disease (CAOD), (2) patients who underwent transthoracic echocardiography (TTE) during their stay in hospital, and (3) patients who underwent carotid ultrasound within 6 months of admission. The exclusion criteria were as follows: (1) underlying disease related to increased cardiac preload (liver cirrhosis or end-stage renal disease), (2) evidence of AF confirmed on electrocardiogram, and (3) moderate to severe valvular regurgitation or any degree of valvular stenosis.
CAOD was defined as Ōēź 50% luminal narrowing of any major coronary artery on the angiogram.
In total, 133 patients satisfied the inclusion criteria, and 104 patients were eventually analyzed in this study after excluding 29 patients. Follow-up data for outcomes were obtained from medical records or telephone interviews when records were not available. This study was approved by the Institutional Review Board of Ewha Medical Center.
Echocardiographic data were obtained from the local medical information system, as recorded by TTE. TTE was performed using one of the three imaging ultrasound systems available in our hospital (iE33, Philips Medical Systems, Bothell, WA, USA; Acuson Sequoia C512, SIEMENS Medical Solution, Malvern, PA, USA; Sonos 5500, Hewlett-Packard Co., Palo Alto, CA, USA). All examinations were carried out by skilled sonographers and were reviewed by two experienced cardiologists.
Left ventricular (LV) mass was calculated using the following formula [6]:
0.80 ├Ś 1.04 ├Ś {(LVEDD + LVST + PWT)3 ŌĆō (LVEDD)3} + 0.6, where LVEDD is the LV end-diastolic diameter, LVST is the LV septal thickness, and PWT is the posterior wall thickness. LV mass was indexed for the body surface area to obtain the LV mass index (LVMi).
Left atrial (LA) diameter was measured using the two-dimensional guided M-mode. LA volume was calculated using the formula for the ellipsoid model [6,12]:
4/3ŽĆ (L/2) (D1/2) (D2/2)
where L is the LA diameter in M-mode, and D1 and D2 are measured from the short and long axes in the apical four-chamber view. LAVi was defined as the ratio of the LAV to body surface area.
Either an 11-mHz (iE33 and Sonos 5500) or an 8-mHz (Acuson) frequency transducer was used for the carotid ultrasound. IMT was measured at a minimum of three points on the far wall at the carotid bulb. Digital captures of three cardiac cycles of the images or still images were stored. A thickness of > 1 mm for local IMT was defined as a plaque [15]. The plaque area was measured between clavicles and the angle of the jaw in the bilateral common, internal, and external carotid arteries in magnified longitudinal views [15]. The sum of all plaques was defined as the carotid total plaque area. Plaque area was assessed by offline processing (NeXus Ltd. version 11.1, Emed Co., Seoul, Korea) with manual tracing of the plaque area. The tracing was performed by an experienced sonographer (4 years of experience) and confirmed by a cardiologist.
The subjectsŌĆÖ baseline characteristics were compared using the chi-square test for categorical variables and analysis of variance or the Kruskal-Wallis test for continuous variables. Values for the continuous variables are expressed as the mean ┬▒ standard deviation. The Cox-proportional hazards model was used to evaluate the relationships of the CHADS2 score, echo parameters, and carotid atherosclerosis with CCV events. We measured cumulative event-free survival using the Kaplan-Meier method and compared unadjusted differences using the log-rank test. The percentage of excess risk explained for the CHADS2 score was calculated using the following formula:
(HR of univariate analysis ŌĆō adjusted HR) / (HR of univariate analysis ŌĆō 1) ├Ś 100 where HR is the hazard ratio.
A p < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using the SPSS version 19.0 (IBM Co., Armonk, NY, USA).
The baseline laboratory and clinical findings for the patients are listed in Table 1. The average age of the 104 subjects was 60.1 years, and the proportion of female patients was 24%. The average baseline blood pressure was 136.2/74.5 mmHg. A history of hypertension was recorded for 40 patients (38.5%) and of diabetes mellitus for 25 patients (24.0%). The average fasting glucose and serum low density lipoprotein cholesterol (LDL-C) levels were 138.2 ┬▒ 49.4 and 121.0 ┬▒ 33.0 mg/dL, respectively. The average CHADS2 score was 0.96 ┬▒ 1.16, and the majority of the patients had a score of 0 or 1 (Table 1).
All patients had ACS; ST elevation myocardial infarction (STEMI) was the most frequent clinical diagnosis in 66 patients (63.5%), followed by non-STEMI in 21 (20.2%) and unstable angina in 17 patients (16.3%) (Table 1).
Baseline characteristics according to the CHADS2 scores are shown in Table 2; age and the presence of diabetes mellitus and hypertension increased with the CHADS2 score by definition. Compared with the group with a CHADS2 score of 0, the other three groups showed high systolic blood pressure and rapid heart rate. There was no significant difference in ACS type between the groups.
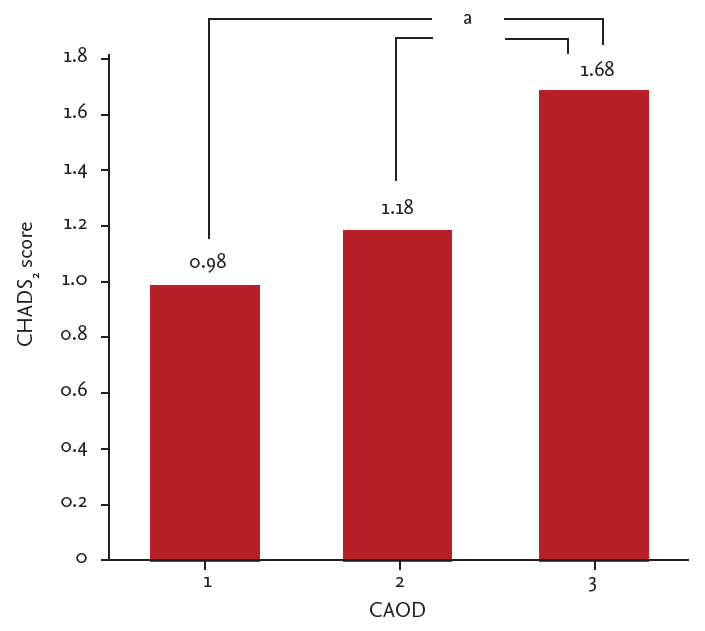
Most patients (53.8%) had single-vessel CAOD. The values for left ventricular ejection fraction (LVEF), LAVi, and LVMi were within the normal ranges (Table 3). The mean right and left IMT was 0.82 ┬▒ 0.26 mm, and the total plaque area was 7.67 ┬▒ 10.59 mm2. Multivessel CAOD showed a positive correlation with increased average carotid IMT and increased total plaque area (1-vessel CAOD vs. 3-vessel CAOD: 0.79 ┬▒ 0.22 mm vs. 0.97 ┬▒ 0.36 mm, p = 0.013 and 5.2 ┬▒ 7.7 mm2 vs. 20.0 ┬▒ 17.5 mm2, p < 0.001, respectively). The severity of CAOD was positively correlated with increasing CHADS2 scores (Fig. 1). Carotid ultrasound revealed that 12 subjects (11.5%) had Ōēź 50% stenosis, and 46 subjects (44.2%) showed evidence of carotid artery calcification. Carotid artery calcification and Ōēź 50% stenosis were both related to the severity of CAOD (p = 0.03 and p < 0.001, respectively). Overall, eight patients (7.6%) showed left main coronary artery disease regardless of the culprit lesion for ACS. LVEF and LAVi showed no relationship with the severity of CAOD (p = 0.63 and p = 0.96, respectively).
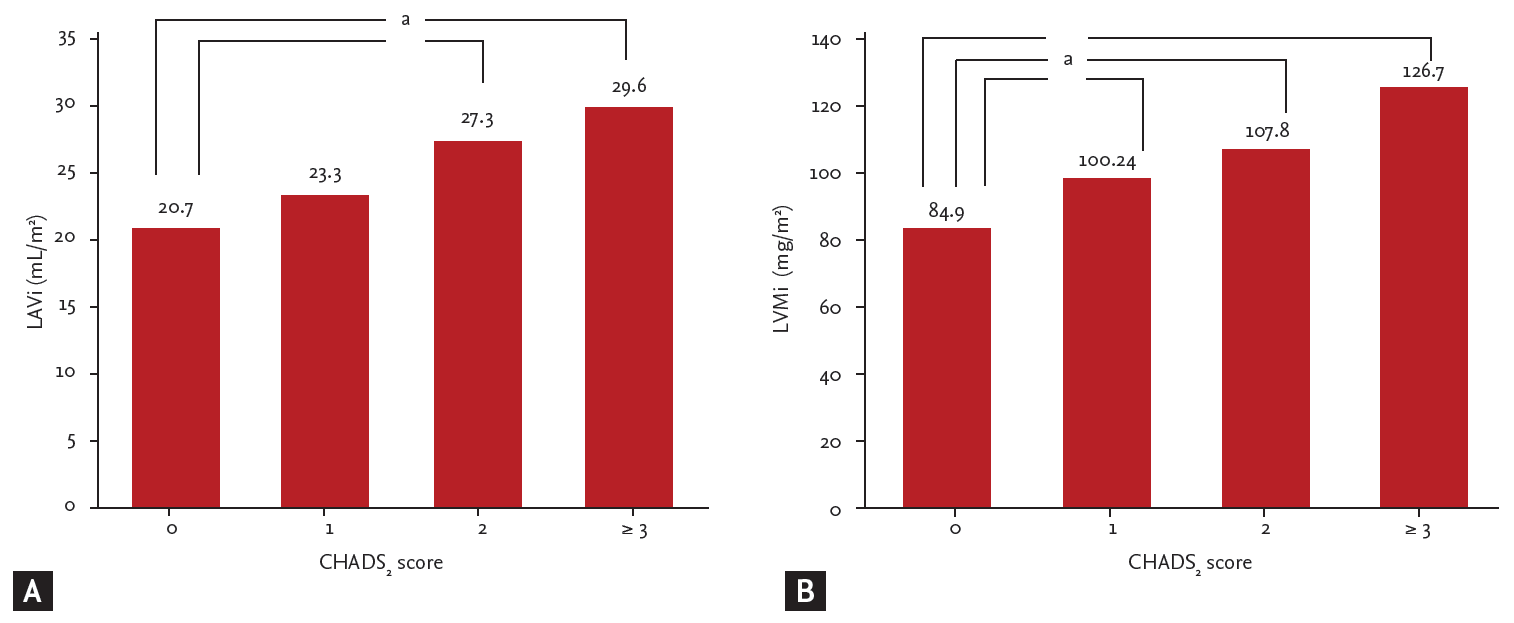
Increased average carotid IMT and plaque area were both related to a CHADS2 score Ōēź 2 (0.77 ┬▒ 0.22 mm vs. 0.97 ┬▒ 0.29 mm, p < 0.001 and 4.97 ┬▒ 7.17 mm2 vs. 15.52 ┬▒ 14.61 mm2, p = 0.002, respectively) (Table 4). An increased CHADS2 score correlated with the presence of carotid artery calcification (p < 0.001) (Table 4). LVMi and LAVi were also positively correlated with the CHADS2 score; these correlations persisted after adjusting for smoking history, systolic blood pressure, sex, body mass index, LDL-C, and creatinine covariates (Fig. 2).
During a mean follow-up period of 662 days, eight cases of CCV events were observed (sudden cardiac death, 1; cerebral infarction, 1; unstable angina, 2; and myocardial infarction, 4). These events were used for the outcome analysis. Other events included new onset of AF (1 case), traumatic subarachnoid hemorrhage (1 case), and cancer-related death (1 case).
Univariate analysis revealed that a CHADS2 score Ōēź 3, CAOD severity, and LAVi were related to an increased risk of CCV events (Table 5). On multivariate analysis, this increased risk remained significant for a CHADS2 score Ōēź 3 and LAVi (model 3).
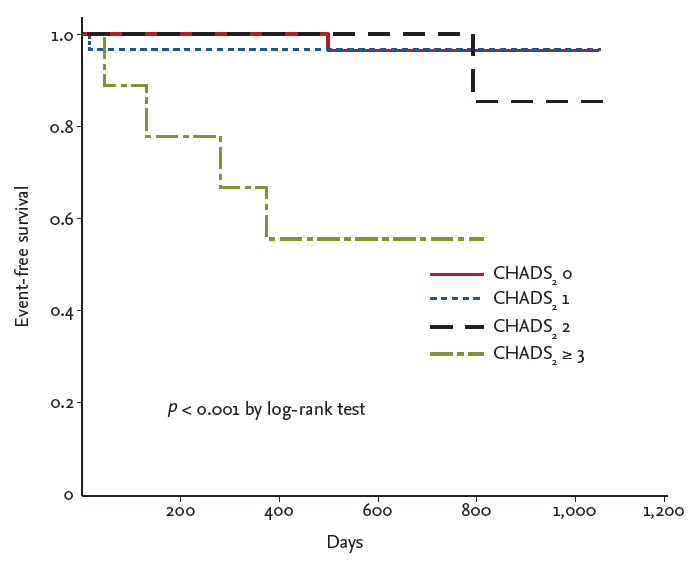
Kaplan-Meier curves were plotted for the CCV outcomes (Fig. 3). A CHADS2 score Ōēź 3 was related to an increased risk of CCV events (p < 0.001 by log-rank test).
The present study demonstrated that the CHADS2 score is related to an increased risk of CCV adverse events in ACS patients with documented coronary artery disease. Furthermore, LAVi was associated with a significant HR in this study. Both LV mass and LAVi have already been established as risk factors for adverse cardiovascular outcomes in the general population as well as in subjects with CHD [13,18-20]. Our data revealed a positive correlation between the CHADS2 score and both LVMi and LAVi. Increased LAVi, i.e., Ōēź 32 mL/m2, has been demonstrated to be a poor prognostic factor [10]. However, in the present study, LAVi showed a positive correlation to the CHADS2 score at values lower than 32 mL/m2 (Fig. 2A). Based on these findings, the CHADS2 score may play a role as an early functional and predictive index for a significant increase in LAVi. In addition, LVMi and LAVi increased steadily with the CHADS2 score prior to changes in other echo parameters. A sudden decrease in left ventricular systolic function was only noted in patients with a CHADS2 score Ōēź 3. CAOD severity was positively correlated with the CHADS2 score in this study. This finding also supports the predictive value of CHADS2 scores for cardiovascular adverse outcomes.
Based on the positive correlations between the CHADS2 score and previously proven predictors of cardiovascular events, LAVi and CAOD severity, it was not difficult to forecast that the CHADS2 score would have a predictive value for cardiovascular events. Our analyses revealed that a CHADS2 score Ōēź 3 was related to an increased risk of CCV events (HR, 14.31). Multivariate analysis (Table 5) revealed that the CHADS2 score continued to be a significant factor after adjusting for the severity of coronary artery disease and LAVi (model 3), with a higher HR than that of LAVi. This result suggests that the CHADS2 score is a stronger risk predictor than other parameters. However, due to the character of the CHADS2 score, which is composed of several clinical variables including congestive heart failure, hypertension, age, diabetes, and stroke, LAVi and the severity of CAOD may actually play a pathophysiological role in CHADS2-related CCV events rather than act as confounding variables. In other words, the predictive value of the CHADS2 score for CCV adverse events was partially due to LAVi and the severity of CAOD as follows: the percentage of excess risk in the CHADS2 score was 27.6% as contributed by LAVi, 26.5% by CAOD severity, and 44.9% by both LAVi and CAOD severity (Table 5).
Another eligible cardiovascular event predictor, as observed in this study, was the carotid total plaque area. The presence of carotid plaques has been reported as a possible predictor of future cardiac death and major cardiovascular adverse outcomes in ACS patients [21,22]. Although there are certain similarities between coronary and carotid arterial disease, reflected as systemic atherosclerosis, ischemic stroke and ACS differ in pathophysiology and can result in different clinical outcomes [17]. In this study, we observed a steep increase in IMT and total plaque area in patients with a CHADS2 score Ōēź 2. However, no significant differences were observed in these parameters between CHADS2 scores of 0 versus 1, 2, or Ōēź 3. Furthermore, no relationship was observed for IMT or total plaque area with CCV adverse events in the Cox-hazard model. Carotid artery calcification and Ōēź 50% stenosis were also not related (data not shown) in the same model. Additionally, we did not find any evidence that IMT, total plaque area, or the presence of carotid plaque were related to CCV outcomes (p = 0.55 by log-rank test). These differences in results may be because our study population differed slightly from those in previous studies; most of our subjects were diagnosed with acute myocardial infarction, with more than half being diagnosed with STEMI. Moreover, the small sample size may have also contributed to this difference.
This study has certain limitations. It included a small sample size and was a single-center study. Furthermore, although we evaluated outcome data, the study was limited by its retrospective, observational nature. The CHADS2 score showed a predictive value for cardiovascular events in patients with ACS and documented coronary artery disease; statistically, the CHADS2 score was a predictive tool for cardiovascular events (area of the receiver operating characteristic [ROC] curve, 0.73; standard error, 0.11; 95% confidence interval, 0.5 to 0.95; p = 0.03) (data not shown). We used a CHADS2 score Ōēź 3 for multivariate analysis based on the Kaplan-Meier survival curve (Fig. 3), which showed markedly decreasing event-free survival. However, a low event incidence (only eight cases) rendered it difficult to confirm the cutoff value using the ROC curve due to low sensitivity. A prospective multicenter registry trial with a large sample population is needed to clarify our results.
In conclusion, our data showed that the CHADS2 score, which is a well-known predictor of stroke in patients with AF, also has a predictive value for CCV events in ACS patients with documented coronary artery disease, regardless of AF. This may be because the CHADS2 score is capable of reflecting the severity of CAOD and early diastolic dysfunction.
1. CHADS2 score may be capable of reflecting the severity of coronary artery obstructive disease and early diastolic dysfunction.
2. CHADS2 score showed a predictive value for cardiovascular and cerebrovascular events in acute coronary syndrome patients with documented coronary artery disease regardless of atrial fibrillation.
Figure┬Ā1.
Relationship between CHADS2 and coronary artery obstructive disease (CAOD). ap < 0.05, adjusted for smoking history, systolic blood pressure, sex, body mass index, low density lipoprotein cholesterol, creatinine.
Figure┬Ā2.
Relationship among (A) left atrial volume index (LAVi), (B) left ventricular mass index (LVMi) and the CHADS2 score (adjusted value). Values presented are adjusted means (with smoking history, systolic blood pressure, sex, body mass index, low density lipoprotein cholesterol, creatinine). ap < 0.05.
Figure┬Ā3.
Kaplan-Meier curves of cardiovascular and cerebrovascular events according to the CHADS2 score.
Table┬Ā1.
Baseline characteristics (n = 104)
Table┬Ā2.
Baseline characteristics by CHADS2 score
| Characteristic |
CHADS2 score |
p value | |||
|---|---|---|---|---|---|
| 0 (n = 46, 44.2%) | 1 (n = 31, 29.8%) | 2 (n = 18, 17.3%) | Ōēź 3 (n = 9, 8.7%) | ||
| Female sex | 5 (10.9) | 7 (22.6) | 9 (50.5) | 4 (44.4) | 0.004 |
| Age, yr | 54.7 ┬▒ 10.0 | 58.2 ┬▒ 10.3 | 71.3 ┬▒ 13.1a,b | 72.0 ┬▒ 10.1a,b | |
| Body mass index, kg/m2 | 24.1 ┬▒ 3.1 | 24.9 ┬▒ 2.7 | 25.1 ┬▒ 3.8a,b | 23.9 ┬▒ 4.5a,b | |
| SBP, mmHg | 127.3 ┬▒ 24.5 | 140.1 ┬▒ 28.7a | 148.7 ┬▒ 28.2a | 144.7 ┬▒ 34.0 | |
| DBP, mmHg | 73.2 ┬▒ 12.3 | 77.2 ┬▒ 15.1 | 72.8 ┬▒ 19.3 | 74.8 ┬▒ 15.5 | |
| Heart rate, beat/min | 67.8 ┬▒ 12.1 | 74.9 ┬▒ 15.4a | 78.7 ┬▒ 14.8a | 78.9 ┬▒ 24.0a | |
| LDL-C, mg/dL | 131.7 ┬▒ 31.5 | 112.7 ┬▒ 28.7 | 121.8 ┬▒ 35.5 | 93.9 ┬▒ 30.9a | |
| Creatinine, mg/dL | 0.98 ┬▒ 0.02 | 0.92 ┬▒ 0.03 | 0.96 ┬▒ 0.07 | 1.1 ┬▒ 0.44 | 0.103 |
| Diabetes mellitus | 0 | 7 (22.6) | 12 (66.7) | 6 (66.7) | < 0.001 |
| Hypertension | 0 | 19 (61.3) | 13 (72.2) | 8 (88.9) | < 0.001 |
| Smoker | 39 (84.5) | 23 (74.2) | 8 (44.4) | 7 (77.8) | 0.002 |
| ACS type | 0.203 | ||||
| ŌĆāUnstable angina | 6 (13.0) | 5 (16.1) | 6 (33.3) | 0 | |
| ŌĆāSTEMI | 33 (71.7) | 17 (54.8) | 9 (50.0) | 7 (77.8) | |
| ŌĆāNSTEMI | 7 (15.2) | 9 (29.0) | 3 (16.7) | 2 (22.2) | |
Table┬Ā3.
Results of coronary angiography and ultrasound examination (n = 104)
Table┬Ā4.
Ultrasound results classified by CHADS2 score
| Variable |
CHADS2 score |
|||
|---|---|---|---|---|
| 0 (n = 46, 44.2%) | 1 (n = 31, 29.8%) | 2 (n = 18, 17.3%) | Ōēź 3 (n = 9, 8.7%) | |
| Carotid ultrasound | ||||
| ŌĆāIMT, mm | 0.73 ┬▒ 0.2 | 0.83 ┬▒ 0.2 | 0.98 ┬▒ 0.2a,b | 0.97 ┬▒ 0.4a |
| ŌĆāTotal plaque area, mm2 | 4.23 ┬▒ 6.8 | 6.03 ┬▒ 7.7 | 15.78 ┬▒ 13.2a,b | 15.01 ┬▒ 18.1a,b |
| ŌĆāCalcificationc | 11 (25.0) | 16 (51.6) | 12 (75.0) | 7 (87.5) |
| ŌĆā> 50% LNc | 2 (4.3) | 2 (6.2) | 6 (35.3) | 2 (22.2) |
| Echocardiogram | ||||
| ŌĆāEF, % | 55.2 ┬▒ 9.6 | 55.7 ┬▒ 12.5 | 60.0 ┬▒ 11.0 | 43.9 ┬▒ 11.7a,b |
| ŌĆāLVMi, mg/m2 | 90.0 ┬▒ 15.6 | 101.9 ┬▒ 20.8a | 107.0 ┬▒ 27.6a | 124.0 ┬▒ 26.8a,b |
| ŌĆāLAVi, mL/m2 | 20.8 ┬▒ 5.9 | 23.2 ┬▒ 6.7 | 26.6 ┬▒ 10.8a | 30.3 ┬▒ 8.3a,b |
| CAOD | 1.3 ┬▒ 0.6b | 1.7 ┬▒ 0.7a | 2.1 ┬▒ 0.8a,b | 2.1 ┬▒ 0.9a |
Table┬Ā5.
Hazard ratios for adverse cardiovascular and cerebrovascular outcomes
REFERENCES
1. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864ŌĆō2870.


2. Welles CC, Whooley MA, Na B, Ganz P, Schiller NB, Turakhia MP. The CHADS2 score predicts ischemic stroke in the absence of atrial fibrillation among subjects with coronary heart disease: data from the Heart and Soul Study. Am Heart J 2011;162:555ŌĆō561.



3. Po├¦i D, Hartford M, Karlsson T, Herlitz J, Edvardsson N, Caidahl K. Role of the CHADS2 score in acute coronary syndromes: risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest 2012;141:1431ŌĆō1440.


4. Hoshino T, Ishizuka K, Shimizu S, Uchiyama S. CHADS2 score predicts functional outcome of stroke in patients with a history of coronary artery disease. J Neurol Sci 2013;331:57ŌĆō60.


5. Sato S, Yazawa Y, Itabashi R, Tsukita K, Fujiwara S, Furui E. Pre-admission CHADS2 score is related to severity and outcome of stroke. J Neurol Sci 2011;307:149ŌĆō152.


6. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of EchocardiographyŌĆÖs Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440ŌĆō1463.


7. Daimon M, Watanabe H, Abe Y, et al. Normal values of echocardiographic parameters in relation to age in a healthy Japanese population: the JAMP study. Circ J 2008;72:1859ŌĆō1866.


8. Connolly HM, Oh JK. Echocardiography. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. BraunwaldŌĆÖs Heart Disease. 9th ed. Vol. 1. Philadelphia: Elsevier, 2012;200ŌĆō276.
9. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107ŌĆō133.


10. Beinart R, Boyko V, Schwammenthal E, et al. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol 2004;44:327ŌĆō334.


11. Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study). Am J Cardiol 2008;102:70ŌĆō76.



12. Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol 2003;41:1036ŌĆō1043.


13. Leung DY, Chi C, Allman C, et al. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol 2010;105:1635ŌĆō1639.


14. Lisowska A, Musia┼é WJ, Lisowski P, Knapp M, Ma┼éyszko J, Dobrzycki S. Intima-media thickness is a useful marker of the extent of coronary artery disease in patients with impaired renal function. Atherosclerosis 2009;202:470ŌĆō475.


15. Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke 2002;33:2916ŌĆō2922.


16. Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis 2012;220:128ŌĆō133.


17. Jashari F, Ibrahimi P, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: similarities and differences. Atherosclerosis 2013;227:193ŌĆō200.


18. Patel DA, Lavie CJ, Milani RV, Ventura HO. Left atrial volume index predictive of mortality independent of left ventricular geometry in a large clinical cohort with preserved ejection fraction. Mayo Clin Proc 2011;86:730ŌĆō737.



19. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561ŌĆō1566.


20. Verma A, Meris A, Skali H, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging 2008;1:582ŌĆō591.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



