 |
 |
| Korean J Intern Med > Volume 30(4); 2015 > Article |
|
To the Editor,
Mad honey poisoning is caused by ingestion of honey made of the nectar from flowers of the rhododendron family [1]. Grayanotoxin is responsible for the clinical picture of intoxication, which is characterized by nausea, vomiting, dizziness, and blurred vision as well as other gastrointestinal, neurological, and cardiac signs and symptoms [12]. We herein report a case involving a 55-year-old woman with grayanotoxin intoxication who presented with chest pain and signs of acute inferior myocardial infarction on electrocardiography after ingestion of mad honey. We also discuss possible mechanisms of the cardiac toxicity associated with this condition.
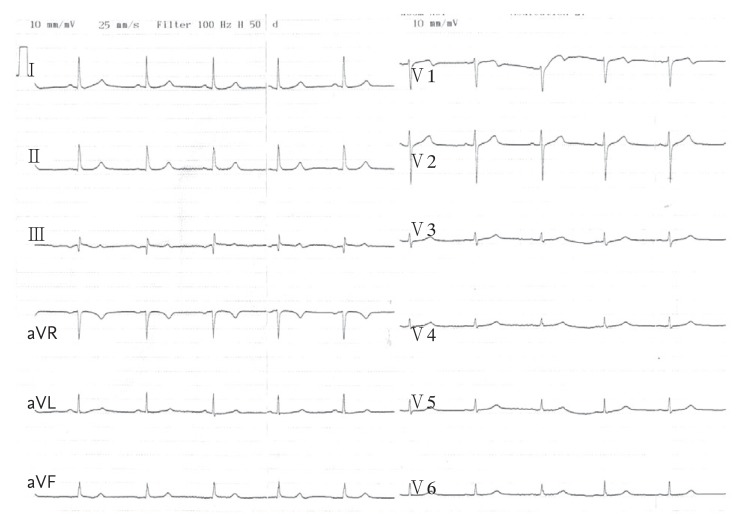
A 55-year-old woman presented to our emergency department with a 5-hour history of chest tightness and pain. She stated that her complaints began after ingesting 3 tablespoons of honey at breakfast that morning. One hour after ingesting the honey, she began to experience nausea and chest tightness that radiated to her left arm. She subsequently experienced weakness, dizziness, and cold shivers. Her medical history was unremarkable except for a thyroid operation to treat goiter. Upon admission, she had a blood pressure of 70/45 mmHg, pulse rate of 46 beats per minute, and oxygen saturation of 95%. Physical examination revealed a short 1/6 systolic murmur at all auscultation points. The remainder of the systemic examination was normal. An electrocardiogram (ECG) showed sinus bradycardia, 1.0-mm ST elevation in leads DIII and aVF, 0.5-mm ST elevation in lead DII, and 1-mm reciprocal ST segment depression in leads DI, aVL, and V3 to V6 (Fig. 1). There were no ST shifts in leads V3R or V4R. Her chest radiograph was normal. Echocardiography showed hypokinesia in the apical portions of the inferior and inferoseptal walls. The creatine kinase-MB and troponin I levels were mildly elevated (32.00 and 0.85 ng/mL, respectively). Based on these findings, the patient was diagnosed with inferior myocardial infarction and treated with 1 mg atropine (one dose), 600 mg clopidogrel, 300 mg acetylsalicylic acid (ASA), and bolus intravenous fluids. She was urgently taken to the catheterization laboratory, where coronary angiography revealed normal epicardial coronary arteries (Fig. 2). None of the three epicardial coronary arteries had thrombi, dissection, haziness, or a myocardial bridge. The patient was given intravenous fluids, ASA, 80 mg atorvastatin, and clopidogrel. With treatment, her blood pressure and pulse rate returned to normal levels within 24 hours. The ST elevation on the ECG returned to baseline (Fig. 3). She was monitored closely in the ward for 3 days and discharged on 100 mg ASA. Her blood pressure and pulse rate were both normal on the third day. Repeat echocardiography showed normal wall motion with an ejection fraction of 60% to 65% and normally functioning heart valves.
Grayanotoxin, also known as andromedotoxin, acetylandromedol, or rhodotoxin [2], is found in the nectar of Rhododendron ponticum, a plant that is endemic to the Black Sea region of Turkey, Nepal, Japan, Brazil, and some regions of North America [3]. In endemic regions, the local inhabitants use grayanotoxin as alternative therapy for various viral infections and gastrointestinal disorders as well as a sexual stimulant. Mad honey can be manufactured and distributed in an uncontrolled fashion in these regions. Our patient bought the honey from an unlicensed local vendor. The cardiotoxic side effects of grayanotoxin arise mainly from an increase in the sodium channel permeability and activation of the vagus nerve. The toxin binds to sodium channels on the cell membrane and increases their permeability, thereby inhibiting repolarization. Consequently, the cell membrane remains depolarized. At the sinus node, the inward sodium current increases and the outward sodium current decreases, attenuating the action potential and causing sinus node dysfunction [34]. Stimulation of the afferent vagal nerve also leads to tonic inhibition of the vasomotor center, resulting in reduced sympathetic output and vagally mediated inhibition of the sinus node [5]. More than 25 grayanotoxins have been identified, among which grayanotoxins 1 and 3 are thought to be the primary toxic isomers. Because we lack the technical means with which to identify grayanotoxins at our institution, we could not determine which of the grayanotoxins had affected our patient.
No factor that might cause hypercoagulability was found in the laboratory analysis. The homocysteine level was normal. No chronic hepatic disease, protein C or S deficiency, or factor V Leiden mutation was detected. Because the patient's symptoms had not begun after stress and no apical ballooning was seen on angiography, we ruled out stress-induced cardiomyopathy. We considered coronary vasospasm as a differential diagnosis. The ergonovine, cold water, and acetylcholine tests were not performed because the patient refused. However, there were no risk factors for coronary spasm, such as smoking, strenuous exercise, psychological stress, or medications that can cause sympathetic vasoconstriction. In addition, the patient's symptoms began after eating honey. Her blood pressure, pulse, and ECG changes improved after the blood pressure and pulse had returned to normal with medical therapy. Consequently, we ruled out coronary vasospasm.
Previous studies have reported various rhythm disorders related to grayanotoxin, including ST elevation, sinus bradycardia, sinoatrial block, QT prolongation, nodal rhythm, and asystole [35]. Our patient exhibited an unusual presentation of grayanotoxin intoxication suggesting acute myocardial infarction. We postulate that the ST elevation and elevated cardiac biomarkers in our patient occurred despite normal coronary arteries and no visible thrombus in the coronary tree as a result of impaired coronary perfusion due to profound hypotension. We believe that the mismatched oxygen supply and demand due to impaired myocardial perfusion led to evolvement of type 2 myocardial infarction.
In conclusion, patients with mad honey poisoning may present with various acute cardiac signs and symptoms, including myocardial infarction. Mad honey ingestion should be considered and appropriate treatment modalities applied in patients presenting with acute cardiac symptoms, including myocardial infarction, in regions where rhododendrons are endemic.
Conflict of Interest
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
2. Jansen SA, Kleerekooper I, Hofman ZL, Kappen IF, Stary-Weinzinger A, van der Heyden MA. Grayanotoxin poisoning: 'mad honey disease' and beyond. Cardiovasc Toxicol 2012;12:208ŌĆō215PMID : 22528814.



3. Yarlioglues M, Akpek M, Ardic I, Elcik D, Sahin O, Kaya MG. Mad-honey sexual activity and acute inferior myocardial infarctions in a married couple. Tex Heart Inst J 2011;38:577ŌĆō580PMID : 22163140.


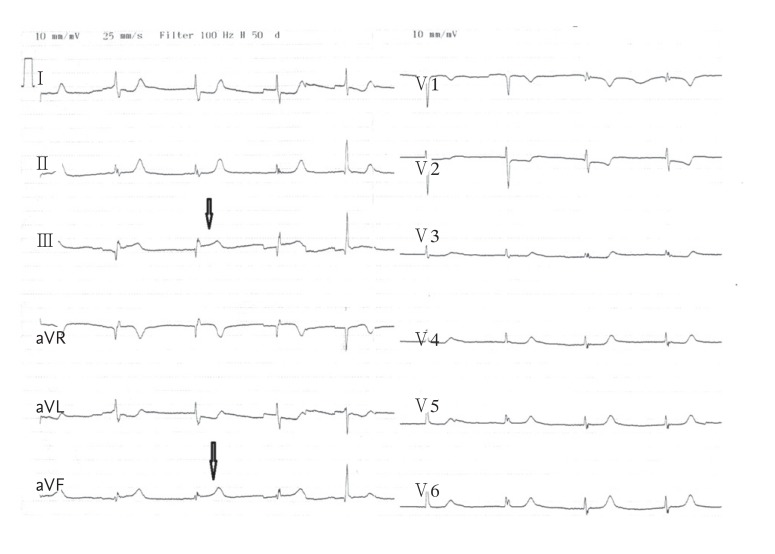
Figure┬Ā1
Electrocardiogram showing ST segment elevation in leads DIII and aVF (arrow) and reciprocal ST segment depression in leads DI, aVL, and V3 to V6.
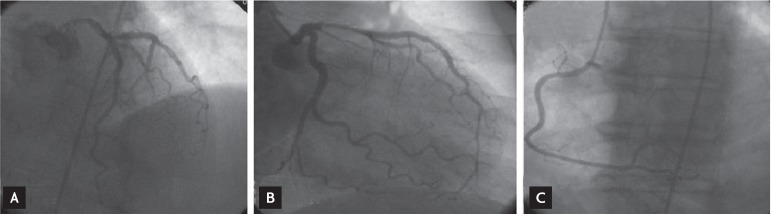
Figure┬Ā2
Normal epicardial coronary arteries on coronary angiography. (A) Left anterior descending and circumflex artery on left anterior oblique cranial projection. (B) Left anterior descending and circumflex artery on right anterior oblique caudal projection. (C) Right coronary artery on left anterior oblique projection.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



