To the Editor,
The prevalence of pancreatic cystic neoplasm (PCN) has been increasing due to advances in diagnostic technology, including ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography and endoscopic ultrasound. When PCN is found incidentally, pancreatic cystic lesions may represent a malignant or premalignant neoplasm and require diagnostic evaluation [1]. Generally, cystic mucin-producing pancreatic neoplasms do not communicate with the pancreatic duct and are classified as benign adenomas or borderline, low-grade malignant and non-invasive or invasive carcinomas according to the grade of epithelial dysplasia. These tumors occur almost exclusively in females aged 50 to 60 years [1]. Mucinous cystic neoplasms (MCNs) are characterized by an ovarian-type stroma that typically forms a band of densely packed spindle-shaped cells beneath the malignant epithelium [1]. Although there are several hypotheses of their origin, the pathogenesis of pancreatic MCNs remains unclear because MCNs are rare and molecular studies are difficult since the tumors often contain only a small number of malignant cells [2].
Clonorchiasis is a parasitic disease common in Far Eastern countries, such as Korea and China. Its symptoms are diverse, although the majority of patients are asymptomatic. The parasite may damage bile duct epithelial cells, causing cholangitis and cholangiocarcinoma. The severity of the disease is proportionate to the number of the infectious parasites and the infection period [3]. Infection with a large number of parasites can result in invasion of the pancreatic duct [3] and the parasites may damage ductal epithelial cells and cause inflammation in the pancreas and the bile duct, leading to clonorchiasis-induced pancreatitis. There are reports associating clonorchiasis and pancreatic malignancies with biliary malignancies, including one case of clonorchiasis-associated pancreatic adenocarcinoma [4]; however, clonorchiasis-associated pancreatic MCN has not been reported.
Here, we report a case of pancreatic mucinous cystadenoma of borderline malignancy infested with Clonorchis sinensis found incidentally in a 53-yearold male with rectal cancer. The patient presented with lower abdominal pain and hematochezia lasting 3 months. The patient often ate freshwater fish and was not a heavy drinker. His medical history was unremarkable except for chronic hepatitis B reactivation treated with 0.5 mg/day entecavir for 1 month. His mother also had chronic hepatitis B patient and succumbed to hepatocellular carcinoma. On admission, the patient's body temperature, heart rate, respiratory rate, and blood pressure were 37.1Ōäā 70/min, 22/min, and 100/60 mmHg, respectively. Physical examination of the neck, chest, and abdomen showed no abnormal findings. Digital rectal examination revealed a non-tender, fixed, hard mass at the posterior rectum, 6 cm from the anal verge. An initial complete blood count revealed a hemoglobin count of 13.3 g/dL, a platelet count of 245,000/┬ĄL, and a white cell count of 5,700/┬ĄL. Biochemical testing showed a blood urea nitrogen of 11 mg/dL, creatinine of 0.6 mg/dL, total protein of 7.1 g/dL, albumin of 3.4 g/dL, aspartate amino transferase of 41 IU/L, alanine transaminase of 37 IU/L, alkaline phosphatase of 69 IU/L, uric acid of 4.4 mg/dL, total calcium of 8.7 mg/dL, phosphorus of 5.1 mg/dL, lactate dehydrogenase of 233 IU/L, carcinoembryonic antigen of 1.4 ng/mL, carbohydrate antigen 19-9 22.0 U/mL, and ╬▒-fetoprotein of 62.9 ng/mL. A chest X-ray evaluation showed no specific findings.
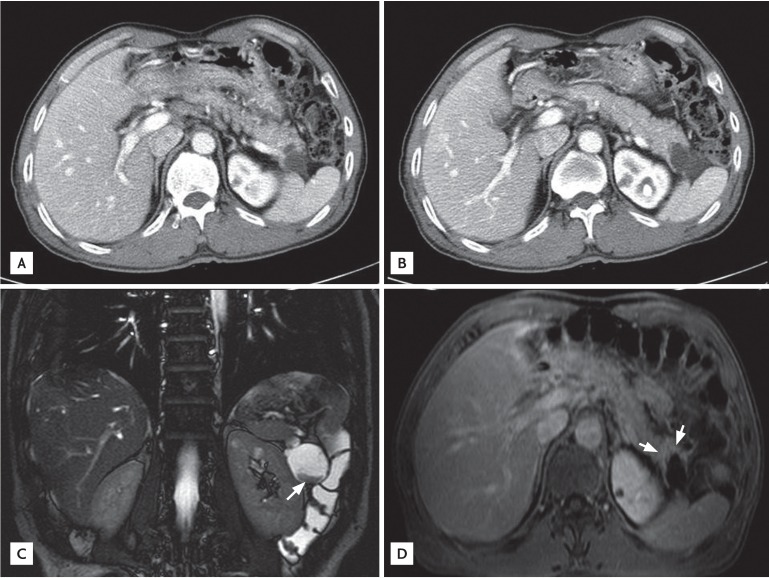
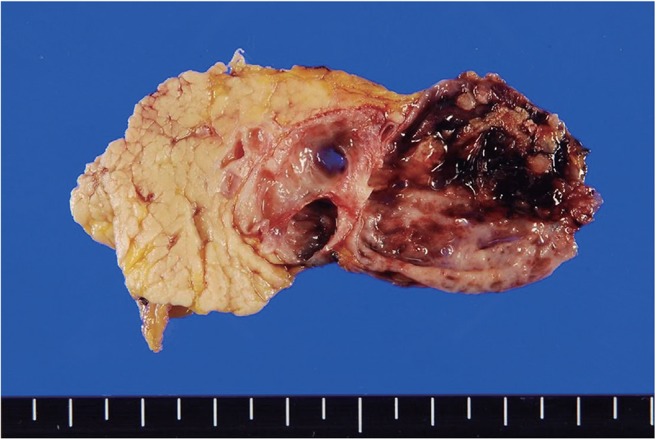
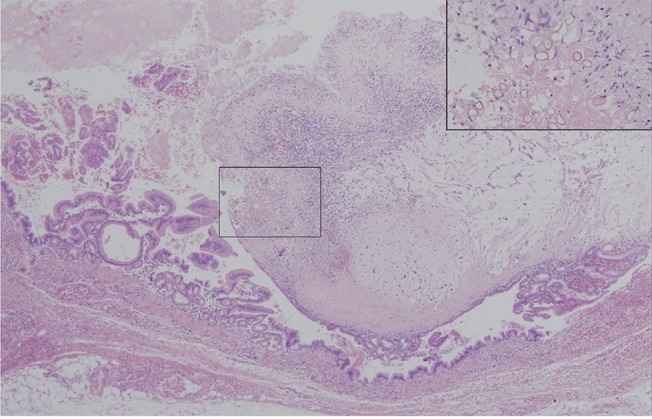
Gastroscopic examination showed no specific abnormality. Colonoscopic examination revealed a large ulcerofungating mass at the distal rectum, and he was diagnosed with adenocarcinoma of the rectum. Abdomen and pelvis CT showed an asymmetric contrast enhancement in the posterior wall of the distal rectum. In addition, a 4.3-cm, heterogeneous, solid and cystic mass on the distal pancreas was found incidentally (Fig. 1A and 1B). Abdominal MRI showed a multi-septated cystic tumor in the pancreas tail and a fibrotic component was found with mild contrast enhancement after gadolinium injection (Fig. 1C and 1D). There was no dilatation of the pancreatic duct in the tail portion, and there was no dilatation of the common bile duct or the intrahepatic bile duct. On day 2 of hospitalization, an ultra-lower anterior resection and distal pancreatectomy was performed. Gross examination of the resected pancreas presented a well-circumscribed cystic mass, measuring 4.4 ├Ś 4.4 ├Ś 3.7 cm. Sectioning revealed a multilocular cyst filled with mucinous and necrotic material (Fig. 2). Microscopically, the multilocular cyst was lined by tall-columnar, mucin-secreting cells with stratification and papillary growth, and mild to moderate nuclear atypia, without stromal invasion. These findings were consistent with a mucinous cystadenoma of borderline malignancy. In addition, there was a papillary growing, nodular lesion embedded in the myxoid and fibrotic stroma with numerous eggs, morphologically considered to be C. sinensis, The eggs were surrounded by epithelioid histiocytes or found within multinucleated giant cells (Fig. 3). The rectal sample obtained from the low anterior resection showed moderately differentiated adenocarcinoma invading the muscle layer, but without lymph node metastasis.
On day 6 after surgery, the patient complained of abdominal pain because of leakage at the surgical area. An exploratory laparotomy was performed and the area washed and drained. After this procedure, the patient showed satisfactory improvement and left the hospital on 15 days after surgery. He is undergoing follow-up care in the Department of Surgery and Hepatology.
Clonorchiasis is caused by eating raw freshwater fish, which are the intermediary hosts of the metacercariae of C. sinensis. The metacercariae is stripped of its cyst by gastric acid, and the larva passes through the ampulla of Vater to mature in the bile duct. Clonorchiasis is associated with cholangitis, biliary stones, and cholangiocarcinoma; the prevalence of clonorchiasis is much higher in patients with cholangiocarcinoma [3]. In Pusan, an area with an extremely high prevalence of C. sinensis, flukes increase the risk of cholangiocarcinoma 6-fold. Animal experiments have also show a strong association between clonorchiasis and cholangiocarcinoma. Therefore, C. sinensis is believed to have malignant potential in the bile duct.
As the larva move to the bile duct, some pass through the main pancreatic duct to branch pancreatic ducts, causing pancreatic disorders [3]. Invasion of the pancreas may result in pancreatitis. Two mechanisms have been proposed by which C. sinensis causes pancreatitis: mechanical obstruction resulting in chemical stimuli by the mixture of the stagnant pancreatic fluid and the metabolites produced by C. sinensis, or inflammation and fibrosis caused by C. sinensis resulting in a back-current of bile into the pancreatic duct [5].
There have been few reports on the association between clonorchiasis and pancreatic neoplasms. A case of pancreatic adenocarcinoma associated with C. sinensis has been reported [4], while cases of clonorchiasiscombined pancreatic MCN have not. In the former reports, an ultrasonogram showed marked dilatation of the intrahepatic and extrahepatic bile ducts. Biopsies of the pancreatic lesion revealed well differentiated ductal adenocarcinoma, but C. sinensis was detected in the common bile duct. Therefore, a direct association between pancreatic adenocarcinoma and C. sinensis could not be proven. However, in this case, C. sinensis was present in a mucinous cystadenoma, suggesting an association between this parasite and MCN. Although parasitic mechanical irritation and chemical injury may be involved in the pathogenesis of pancreatic mucinous cystadenoma by inducing molecular changes, similar to clonorchiasis-associated cholangiocarcinoma, we could not determine pancreatic ductal dilation and inflammatory changes, representing mechanical obstruction, and parasitic irritations. Therefore, we concluded that C. sinensis was associated with pancreatic mucinous neoplasm.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





