INTRODUCTION
Immunoglobulin A nephropathy (IgAN) is the most common cause of primary glomerulonephritis worldwide and a frequent cause of end-stage renal disease (ESRD) in patients with primary glomerular disease [
1]. Glomeruli typically contain generalized diffuse granular mesangial deposits of IgA. IgAN is generally regarded as an immune complex-mediated glomerulonephritis. The characteristic presentation is gross hematuria at the time of an infectious episode. Patients with IgAN have a variable clinical course, from a benign condition to progressive deterioration in kidney function over time. However, in many patients, disease progression is slow, with 14% to 39% of patients developing ESRD over a 20year period after diagnosis [
2]. Previous studies have identified several potential clinical predictors for progression, including degree of renal impairment at diagnosis, proteinuria, albumin level, high blood pressure at presentation, age, and male gender [
3,
4,
5]. Several histological features have also been shown to be associated with a progressive course. However, prediction of renal outcome in an individual patient remains difficult. For decisions regarding the course of treatment, more accurate prognostic predictors are needed.
Several recent studies on early predictive biomarkers of both acute kidney injury (AKI) and chronic kidney disease (CKD) have been reported [
6]. Among them, neutrophil gelatinase-associated lipocalin (NGAL) is one of the most promising novel biomarkers. NGAL is a small (25 kDa) protein belonging to the lipocalin family, which is released from kidney tubular cells after harmful stimuli [
7]. After renal injury, NGAL is increased significantly in human kidney cortical tubules, blood, and urine [
8]. NGAL has been identified as an early predictive biomarker for AKI [
9]. However, recent studies have also suggested a possible role for NGAL in CKD [
10,
11]. In CKD, the rate of deterioration in renal function and adverse renal outcomes are associated with the degree of renal tubulointerstitial impairment rather than with the severity of glomerular lesions [
12]. Involvement of several tubular proteins in the pathogenesis of tubular damage has been reported [
13]. NGAL has been shown to represent a biomarker for degree of tubulointerstitial damage in patients with CKD [
14,
15].
We hypothesized that NGAL could be used as a new prognostic predictor with its potential role in tubular injury in IgAN. Thus, we attempted to determine whether plasma NGAL (pNGAL) was associated with other clinical prognostic factors and a potential biomarker for predicting outcomes in patients with IgAN.
METHODS
Subjects
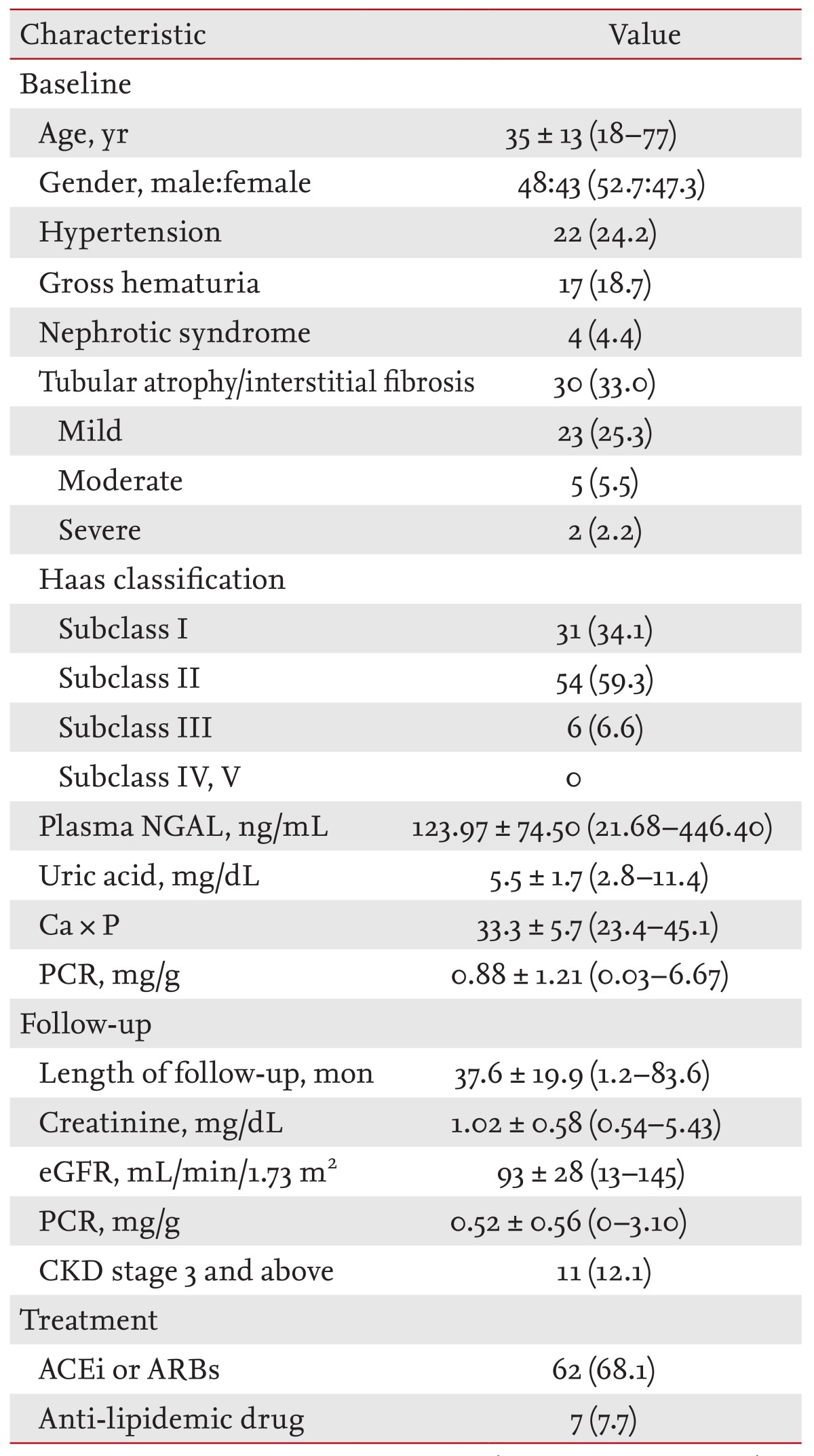
Among 406 patients diagnosed with biopsy-proven IgAN in the Department of Internal Medicine, Kyungpook National University, from February 2002 to August 2010, we examined 91 patients with available samples for analysis and clinical data. The study was approved by the local Ethics Committee (IRB No. 2013-04-039). All patients gave written informed consent.
Patients with cancer, infection, or autoimmune disease were excluded to avoid potential confounding factors. The medical records were reviewed retrospectively and clinical data, including age, gender, presence of hypertension (HTN), histological findings, and treatment methods at the time of renal biopsy, were recorded. Tubulointerstitial change was classified as absent (0%), mild (1% to 25%), moderate (26% to 50%), or severe (> 50%). Mild or above was defined as tubular atrophy/interstitial fibrosis. The histological assessment of renal biopsies was performed according to the Haas classification. Haas subclass III or above was defined as higher histological grade. CKD stage 3 or above was defined as an adverse renal outcome.
Laboratory measurements
Common biochemical parameters, including urea, creatinine, uric acid, serum lipids, electrolytes, albumin, hemoglobin, and proteinuria, were measured at baseline in all patients, according to standard methods in the routine clinical laboratory. Additional blood samples were stored at -80Ōäā until assayed.
At the time of renal biopsy, pNGAL was measured in all patients using a commercially available enzyme-linked immunosorbent assay test kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. Microplate wells were coated with a monoclonal antibody specific for NGAL. Standards and samples were pipetted into wells and any NGAL present was bound by the immobilized antibody. After incubation, the unbound conjugate was washed off and substrate solution was added to each well. The intensity of color developed was proportional to the concentration of NGAL in the patient plasma sample. pNGAL levels were expressed in nanograms per milliliter and the minimum detectable level was 0.012 ng/mL.
Statistical analysis
Data are reported as means ┬▒ standard deviation, median and interquartile range, or percentage frequency, as appropriate, and
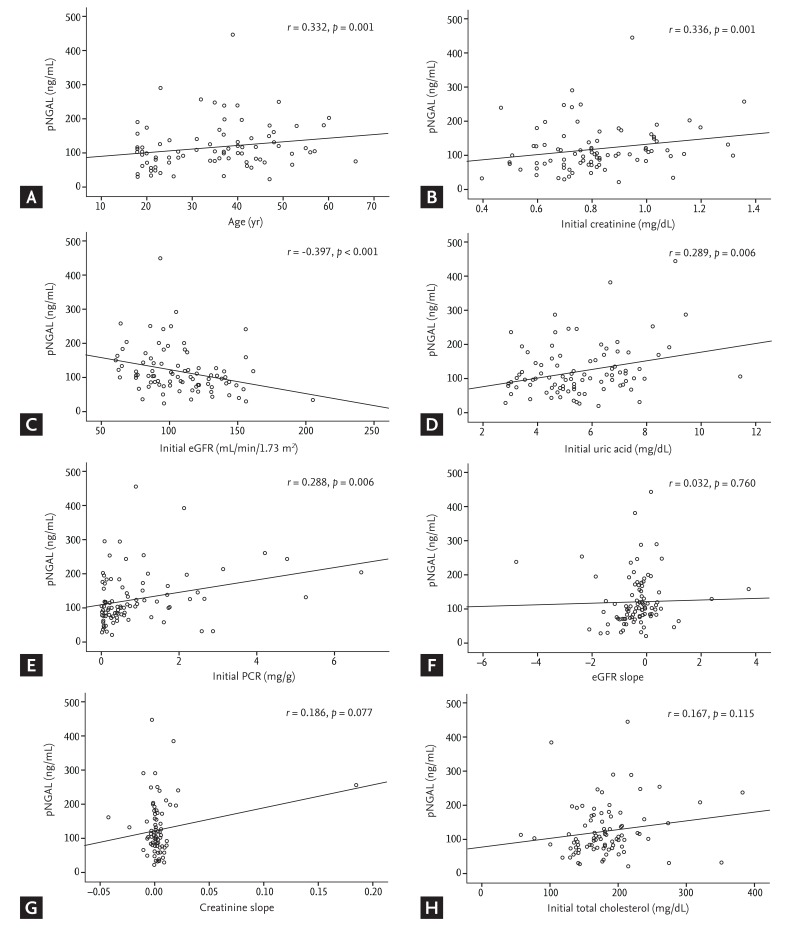
t tests or one-way analysis of variance was used for comparisons among groups. Correlations between pNGAL and age, initial creatinine, estimated glomerular filtration rate (eGFR), uric acid, urinary protein-to-creatinine ratio (PCR), eGFR slope, and creatinine slope were assessed using Pearson correlation coefficients. eGFR is estimated GFR, calculated by the abbreviated Modification of Diet in Renal Disease equation:
The predictive value for renal outcome was analyzed by receiver operating characteristic (ROC) curve analysis, and the area under the curve (AUC) the ROC curve was calculated. Univariate and multivariate Cox regression analysis were performed for analysis of the association between clinical parameters and renal outcome. Statistical analyses were performed using the SPSS version 19.0 (IBM Co., Armonk, NY, USA). Results were considered significant if p < 0.05.
DISCUSSION
In this study, we demonstrated that pNGAL in patients with IgAN was significantly higher in patients with adverse renal outcome and showed strong correlations with tubular atrophy/interstitial fibrosis and other clinical variables. As an independent predictor, pNGAL, when added to well-known predictive factors, including proteinuria and HTN, improved the predictive power for adverse outcomes. To our knowledge, this is the first reported study on the prognostic value and the correlation of pNGAL with other clinical variables in IgAN.
IgAN is a progressive disease with a highly variable clinical presentation and renal outcome [
16]. No optimal treatment for IgAN has yet been established. General treatments, such as control of blood pressure with ACEi or ARBs, and statin therapy are generally administered to patients with IgAN to slow progression, while immunosuppressive therapy is used in selected patients. Indications for immunosuppressive therapy are based on the perceived risk factors for progressive kidney disease. Such patients are more likely to benefit from early or more aggressive therapy [
3,
17]. Previous studies have identified clinical and pathological features associated with adverse renal outcomes. These include heavy proteinuria, reduced renal function, HTN at the time of diagnosis, interstitial fibrosis, and glomerular sclerosis [
18,
19]. However, the overall predictive value of these variables is relatively low. Thus, more accurate prognostic tools are needed to more accurately predict the course of IgAN.
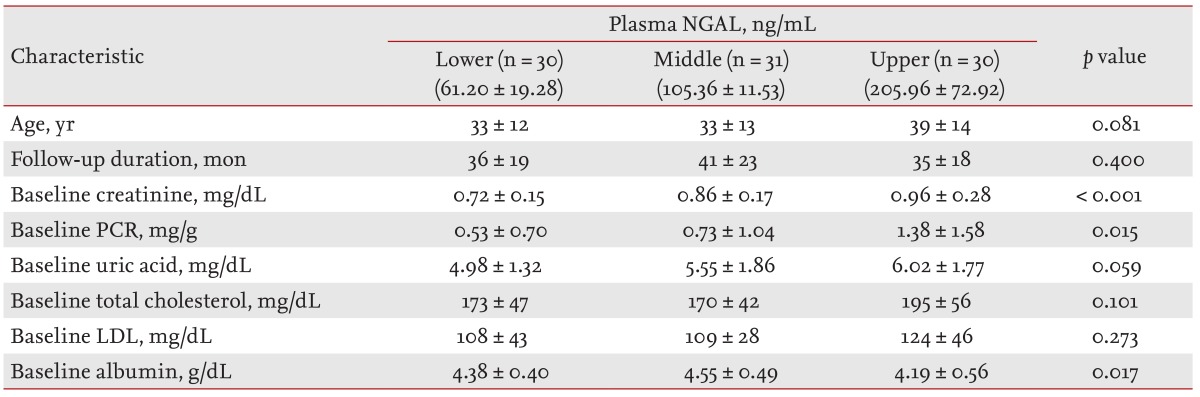
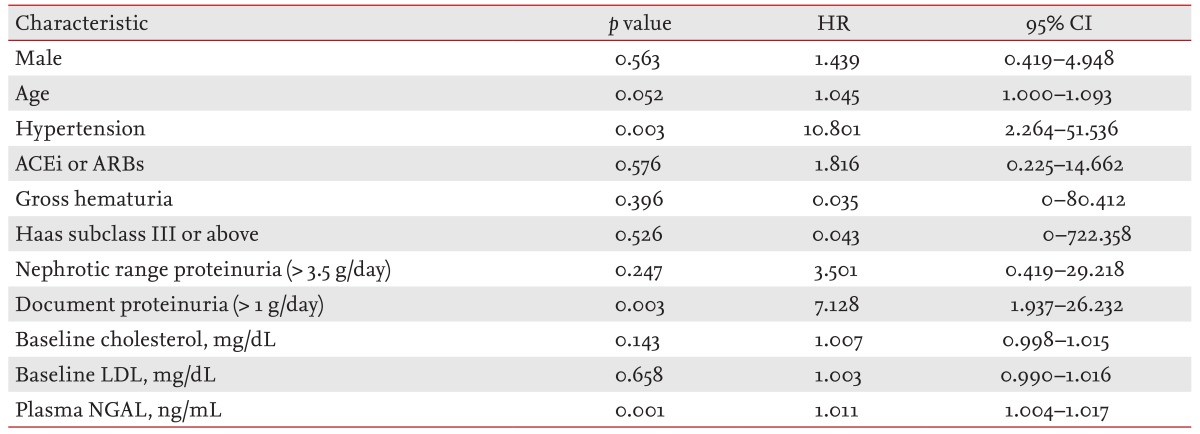
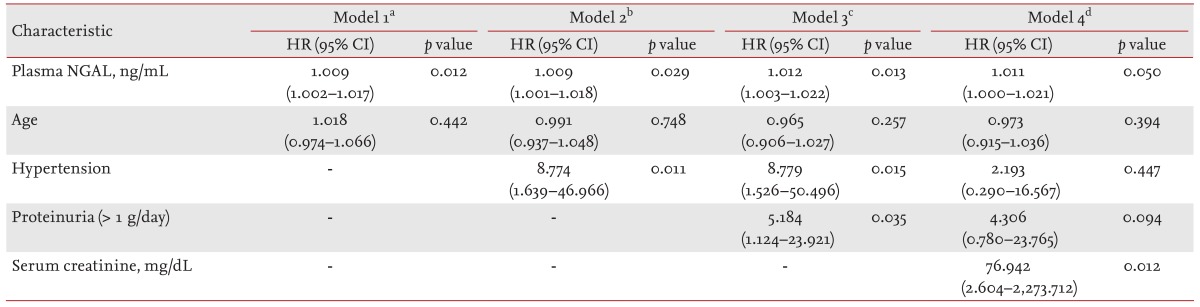
In our study, because adverse renal outcome was defined as CKD stage 3 or above, initial serum creatinine was a strong prognostic factor. pNGAL also showed a positive association with initial creatinine (r = 0.336, p = 0.001). For these reasons, in multivariate analysis including serum creatinine, the variables did not show statistical significance. However, in multivariate analysis except serum creatinine, HTN, proteinuria > 1 g/day, and pNGAL were independent predictors of adverse renal outcome.
An increase in NGAL levels is a good predictor of the short-term onset of AKI, notably anticipating the resulting increase in serum creatinine levels. In parallel, recent studies have also reported altered NGAL levels in patients affected by CKD [
10,
11,
12]. However, fewer studies on the association between NGAL and adverse renal outcome in IgAN have been reported. Ding et al. [
20] reported that urinary NGAL was an early biomarker for renal tubulointerstitial injury in IgAN. Consistently, another study by Peters et al. [
21] reported urinary kidney injury molecule-1 (KIM-1) and NGAL were independent predictors of ESRD in patients with IgAN. However, urinary KIM-1 and NGAL did not show correlations with renal function, and only KIM-1 was an independent predictor of ESRD. This is consistent with several recent reports showing correlations of NGAL with serum creatinine, eGFR, urinary PCR, and tubular atrophy [
10,
11,
12,
21]. In this study, pNGAL also showed strong correlations with age, creatinine, eGFR, uric acid, urinary PCR, and albumin.
Histological features identified as independent predictors of adverse renal outcome included glomerulosclerosis, tubular atrophy, interstitial fibrosis, and mesangial hypercellularity in IgAN [
22,
23,
24]. In this study, pNGAL concentration showed a good correlation with tubular atrophy/interstitial fibrosis. Similarly, pNGAL has been shown to represent a biomarker for degree of tubulointerstitial damage in patients with CKD [
14,
15]. Thus, pNGAL concentration could be a valuable indicator for tubular atrophy/interstitial fibrosis in IgAN, especially in a situation where a renal biopsy could not be performed.
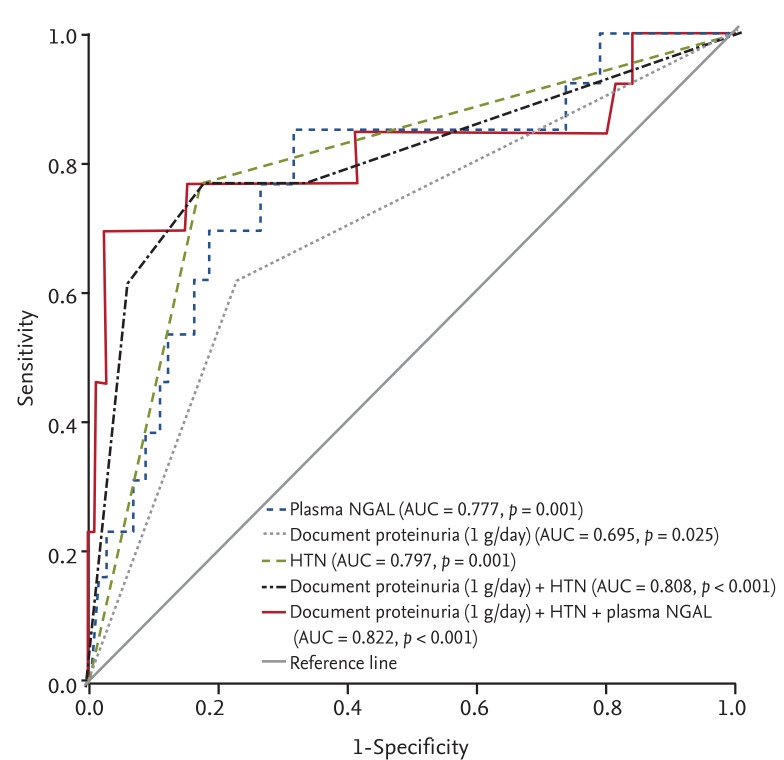
Using ROC curve analysis, pNGAL was found to be a strong prognostic factor for adverse renal outcome. As the predictive power of individual risk factors in IgAN is low, several clinical studies have suggested combining them to increase the predictive power versus individual risk factors [
2,
3,
25]. In this study, the predictive power of pNGAL for adverse renal outcome showed considerable improvement when it was added to well-known clinical predictors, such as proteinuria and HTN. Thus, pNGAL could be used as a clinically relevant risk factor to increase predictive power for adverse outcomes in IgAN.
Although, the current study was somewhat limited by its retrospective design and also underpowered with small numbers of patients and short duration of follow-up period, our study is important because pNGAL was significantly higher in patients with adverse renal outcomes and showed strong correlations with tubular atrophy/interstitial fibrosis and other clinical variables. The clinical applicability of these findings needs to be confirmed in a larger cohort. In this analysis, adjustment for initial serum creatinine could not be performed.
In summary, pNGAL may be useful as a potential predictor of prognosis for IgAN with other clinical parameters and also as a diagnostic indicator of tubular atrophy/interstitial fibrosis in IgAN. Further studies in larger populations are required to evaluate the potential utility of NGAL measurements in the monitoring of disease progression in IgAN.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





