 |
 |
| Korean J Intern Med > Volume 29(5); 2014 > Article |
|
To the Editor,
Primary aldosteronism was originally described by an aldosterone-producing adenoma. Later, primary aldosteronism was recognized to occur as a result of a heterogeneous group of disorders, including adenoma, hyperplasia, or aldosterone- and cortisol-co-producing tumors. Here, we report a rare case of an aldosterone- and cortisol-co-producing adenoma in the presence of primary aldosteronism without the clinical features of Cushing syndrome.
A 29-year-old woman with hypertension presented with seizure and lower extremity weakness. The patient was 166 cm tall, weighed 64.5 kg, and her blood pressure was 165/97 mmHg. No features of Cushing syndrome, such as moon face, purple striae, hirsutism, or central obesity, were evident. Laboratory data showed sodium, 145 mEq/L; potassium, 1.5 mEq/L; chloride, 94 mEq/L; calcium, 8.3 mg/dL; phosphorus, 2.4 mg/dL; magnesium, 2.0 mg/dL; blood urea nitrogen, 6.5 mg/dL; and serum creatinine, 0.5 mg/dL. Spot urine potassium was 37.6 mEq/L, and the transtubular potassium gradient was 14%. Spot urine chloride was 90.2 mEq/L, and a blood gas analysis showed pH, 7.6; pCO2, 42.5 mmHg; pO2, 85 mmHg; HCO3, 44.2 mmol/L; and SpO2, 98%. Thyroid function tests were normal. Fasting plasma glucose and glycated hemoglobin were 96 mg/dL and 4.9%, respectively. Electrocardiography, chest anteroposterior views, brain computed tomography (CT), electroencephalography, and a cerebral spinal fluid study revealed normal findings.
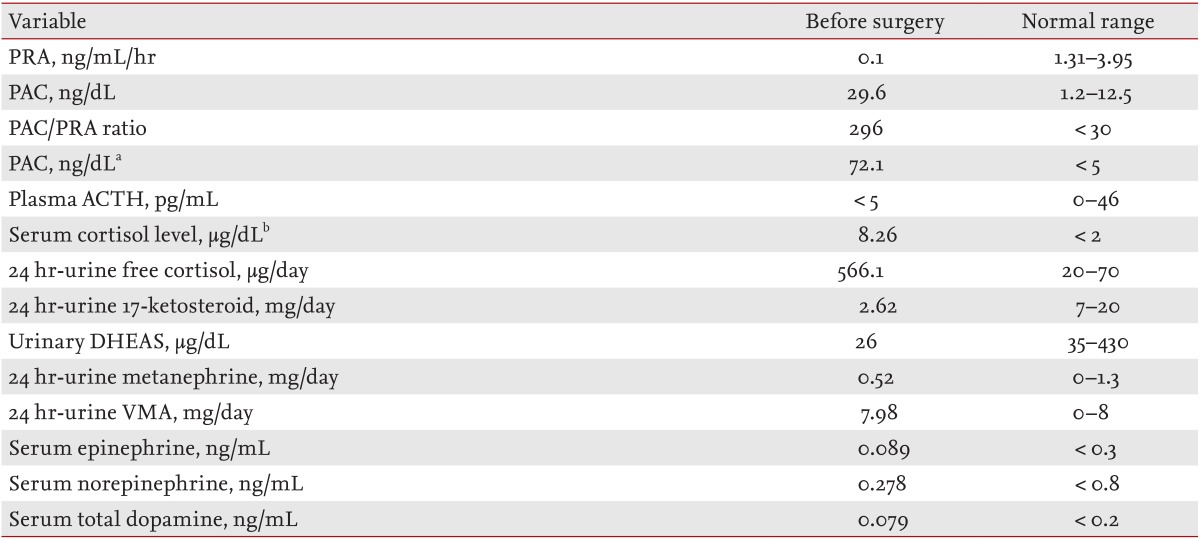
As shown in Table 1, hormone studies showed an increased plasma aldosterone concentration (PAC; 29.6 ng/dL), suppressed plasma renin activity (PRA; 0.1 ng/mL/hr), and an increased PAC:PRA ratio (296), suggesting excess aldosterone. Basal PRA and PAC on the saline infusion test were 0.1 ng/mL/hr and 94.5 ng/dL, respectively. After infusion of 2 L of 0.9% saline over 4 hours, PAC was not suppressed (72.1 ng/dL). The 24-hour urinary free cortisol excretion increased to 566.1 µg/day. Serum cortisol levels were not suppressed with administration of doses of 1 (overnight), 2 (low dose), and 8 mg (high dose) dexamethasone, which were in concentrations of 8.26, 9.54, and 7.79 µg/dL, respectively. Plasma adrenocorticotrophic hormone (ACTH) was < 5 pg/mL, suggesting an ACTH-independent type of Cushing syndrome (Table 1).
An adrenal CT scan revealed a 2.1 × 1.6 cm diameter, well circumscribed, homogeneous, low density nodule in the left adrenal gland. PRA was 0.16 ng/mL/hr, PAC was 54.9 ng/dL, and ACTH was 11.0 pg/mL in the supine position. After 4 hours of standing, PRA was 1.23 ng/mL/hr, PAC was 46.7 ng/dL, and ACTH was < 5 pg/mL. These results suggested an adrenal adenoma rather than bilateral adrenal hyperplasia due to the paradoxical drop in aldosterone with standing. We excluded adrenocortical carcinoma and pheochromocytoma based on the results of hormone studies (Table 1). We could not perform bilateral adrenal venous sampling or 131I-iodocholesterol scintigraphy due to patient refusal.
We diagnosed this case as an aldosterone- and cortisol-co-producing adrenal adenoma based on the very high PAC, cortisol that was not suppressed by dexamethasone, and the adrenal mass on CT. Preoperative management involved 100 mg spironolactone daily and a potassium correction. Subsequently, a laparoscopic left adrenalectomy was performed. The cut surface of the tumor was homogeneous and golden yellow without necrotic features. A histological examination revealed that the adrenocortical adenoma was composed of both clear and compact cells. We performed immunohistochemistry for 3β-hydroxysteroid dehydrogenase (HSD-3β1) and cytochrome P450-17A1 using anti-HSD3β1 (1:50; Abcam, Cambridge, MA, USA) and anticytochrome P450-17A1 (1:50; Abcam), respectively, which suggested that the adrenal adenoma possibly produced both aldosterone and cortisol (Fig. 1).
The patient's hypertension and hypokalemia improved following the adrenalectomy. Just after the surgery, we injected 100 mg hydrocortisone intravenously to prevent an adrenal crisis and then tapered the oral prednisolone for 5 days. After surgery, hormone studies showed normalized PAC (3.1 ng/dL), PRA (0.32 ng/mL/hr), PAC:PRA ratio (6.68), and 24-hour urinary free cortisol excretion (60.81 µg/day). The serum cortisol level after administration of a 1 mg dexamethasone dose (overnight) was 1.93 µg/dL. Ten days after surgery, we performed a rapid ACTH stimulation test, which demonstrated insufficient cortisol secretion. The preinjection plasma ACTH and cortisol levels were 29.6 pg/mL and 5.06 µg/dL, respectively. Plasma cortisol levels 30 and 60 minutes after a single intravenous injection of 250 µg tetracosactide (Synacthen [Alliance Pharma, Chippenham, UK], synthetic ACTH) were 8.14 and 8.94 µg/dL, respectively, indicating adrenal insufficiency. We restarted oral prednisolone at a daily dose of 7.5 mg and tapered it over 5 months. After the patient decided to stop oral prednisolone 5 months after surgery, we confirmed normal cortisol secretion using the rapid ACTH stimulation test.
Several clinical implications are important for aldosterone- and cortisol-co-producing tumors. First, these patients are associated with an increased risk of cardiovascular events, as the incidence of myocardial infarction, heart failure, and stroke increase [1]. Second, there is increased risk for metabolic complications such as glucose intolerance and hypertension [2]. Third, these tumors can lead to decreased bone mineral density and bone quality and an increased risk of fracture [3]. Fourth, cortisol co-secretion may cause false-negative results in adrenal venous sampling [4]. Finally, postoperative adrenal crisis and adrenal insufficiency should be considered in the clinical management [5].
The prevalence of aldosterone- and cortisol-co-producing tumors is not exact. Clinicians should consider a cortisol co-secreting tumor in primary aldosteronism if cortisol is not suppressed by dexamethasone or an adenoma > 2.5 cm is present [4]. Treatment for an aldosterone- and cortisol-co-producing tumor is surgical excision. It should be noted that hydrocortisone replacement is necessary during and after adrenalectomy. In conclusion, we present this case to emphasize that an adrenal adenoma might be capable of secreting both aldosterone and cortisol without clinical features of Cushing syndrome. Therefore, the screening test for Cushing syndrome should be performed in patients with primary aldosteronism even if there are no clinical features of Cushing syndrome.
References
1. Nakajima Y, Yamada M, Taguchi R, et al. Cardiovascular complications of patients with aldosteronism associated with autonomous cortisol secretion. J Clin Endocrinol Metab 2011;96:2512–2518PMID : 21593113.


2. Abdelmannan D, Aron DC. Adrenal incidentalomas and subclinical Cushing's syndrome. Rev Endocr Metab Disord 2010;11:135–140PMID : 20714806.


3. Chiodini I, Morelli V, Masserini B, et al. Bone mineral density, prevalence of vertebral fractures, and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian multicenter study. J Clin Endocrinol Metab 2009;94:3207–3214PMID : 19549741.


Figure 1
In the immunohistochemical findings, the compact cells showed intense expression of cytochrome P450-17A1, implicating the compact cells as the main source of cortisol production (A, ×400, arrows). The clear cells showed expression of 3β-hydroxysteroid dehydrogenase, implicating the clear cells as the main source of aldosterone production (B, ×400, arrows).

-
METRICS

- Related articles
-
Aldosterone-Producing Adrenocortical Carcinoma without Hypertension2012 June;27(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


