 |
 |
| Korean J Intern Med > Volume 29(3); 2014 > Article |
|
Abstract
Background/Aims
In Asia, the incidence of non-Hodgkin lymphoma (NHL) has increased in recent decades. Waldeyer's ring (WR) is the most common site of NHL involving the head and neck. In this study, the pathological distribution of WR-NHL and its clinical features were analyzed retrospectively.
Methods
From January 2000 through December 2010, we analyzed the medical records of 328 patients from nine Korean institutions who were diagnosed with WR-NHL.
Results
The study group comprised 197 male and 131 female patients with a median age of 58 years (range, 14 to 89). The rate of localized disease (stage I/II) was 64.9%, and that of low-risk disease (low/low-intermediate, as defined by the International Prognostic Index) was 76.8%. Diffuse large B-cell lymphoma (DLBCL; 240 patients, 73.2%) was the most common pathologic subtype, followed by peripheral T-cell lymphoma (14 patients, 4.3%) and nasal NK/T-cell lymphoma (14 patients, 4.3%). WR-NHL occurred most frequently in the tonsils (199 patients, 60.6%). Extranodal involvement was greater with the T-cell subtype (20 patients, 42.5%) compared with the B-cell subtype (69 patients, 24.5%). Multivariate analyses showed that age Ōēź 62 years, T-cell subtype, and failure to achieve complete remission were significant risk factors for overall survival.
Conclusions
DLBCL was found to have a higher incidence in Korea than those incidences reported by other WR-NHL studies. T-cell lymphoma occurred more frequently than did follicular lymphoma. T-cell subtype, age Ōēź 62 years, and complete remission failure after first-line treatment were significant poor prognostic factors for overall survival according to the multivariate analysis.
The prevalence of non-Hodgkin lymphoma (NHL) has increased in the last decade [1]. Immune-suppression, genetics, and exposure to chemical agents have contributed to the increasing incidence of NHL [2,3,4]. NHL is not limited to lymph node progression; in contrast to Hodgkin lymphoma, it may arise from or involve extranodal organs in approximately one-third of cases. Waldeyer's ring (WR) is the most popular site of involvement among NHLs presenting in the head and neck. WR originates from lymphoid tissues surrounding the digestive and respiratory systems, including those around the Eustachian tube, upper palatine tonsils, nasopharynx, oropharynx, salivary glands, and sublingual sites. In Asia, NHL involving WR (WR-NHL) has increased recently [5,6,7,8,9,10].
The increased understanding of the pathology and physiology of lymphoma has led to changes in its classification. The World Health Organization (WHO) classification of lymphomas was revised in 2001 and again in 2008 [11,12]. However, recent studies of lymphomas involving WR were performed in few lymphoma subtypes and under limited circumstances. Therefore, those studies were limited in their ability to determine the overall characteristics of WR-NHL. In this study, the clinical characteristics and pathological distribution of WR-NHL in Korea were analyzed retrospectively according to the 2001 and 2008 WHO lymphoma classifications.
From January 2000 through December 2010, 328 Korean patients pathologically confirmed as WR-NHL from nine independent institutions were reviewed retrospectively. Pathologic diagnosis was determined according to the 2001 and 2008 WHO classifications of lymphomas [11,12]. Patients were classified by the Ann Arbor staging system according to the results of computed tomography (CT) scans, positron emission tomography (PET)-CT, bone marrow biopsy, and cerebrospinal fluid analysis if necessary. Clinical and laboratory parameters including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, and biochemical laboratory results were evaluated. The disease stage and extranodal involvement were also investigated.
Patients with localized disease were classified into five groups according to their treatment: 1) supportive care; 2) chemotherapy alone; 3) chemotherapy plus radiotherapy; 4) radiotherapy alone; and 5) surgical resection. The surgical resection group included patients who underwent chemotherapy or radiotherapy after surgery. We evaluated the treatment results and survival of each group using serial CT scans.
Progression-free survival (PFS), disease-free survival (DFS), and overall survival (OS) were analyzed using the Kaplan-Meier and the Cox proportional regression methods. PFS was calculated as the period from the first day of treatment to the date of disease progression or death from any cause. DFS was calculated as the period from the date of complete remission (CR) to that of relapse or death while in CR. OS was calculated as the period from the first day of treatment to the date of death from any cause. The results were expressed as means with 95% confidence intervals (CIs) where appropriate, and p < 0.05 was considered indicative of statistical significance. Statistical analysis was performed using the PASW version 18.0 (SPSS Inc., Chicago, IL, USA).
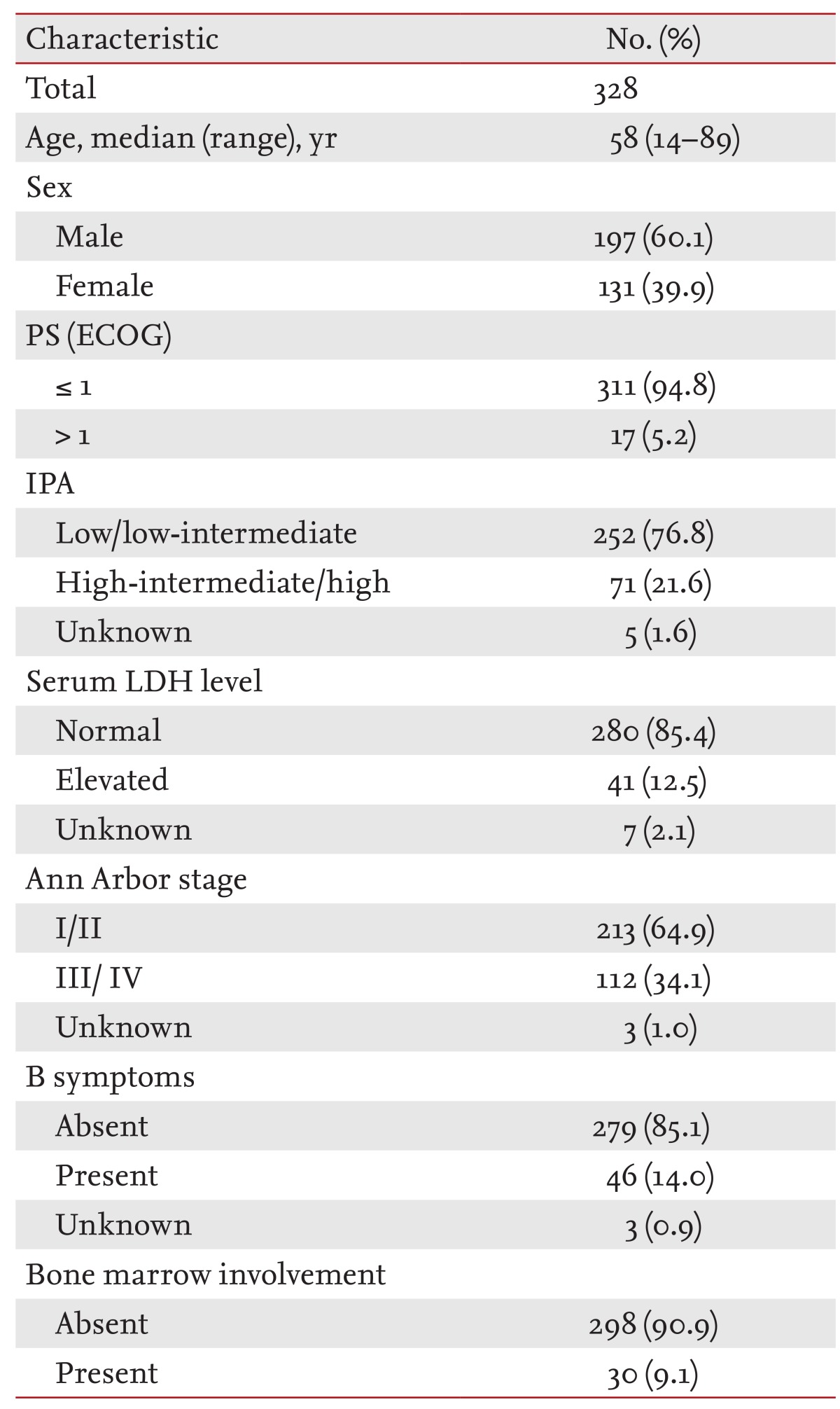
The median patient age was 58 years (range, 14 to 89). The male:female ratio of the 328 patients was 1.5:1. More than half of the patients (64.9%) presented with localized disease (Ann Arbor stage I or II). In particular, the majority of B-cell lineage NHL cases presented as localized disease (71.1%). T-cell lineage NHL cases more frequently showed disseminated disease (57%) compared with other NHLs. Most patients were in the low/low-intermediate risk group (252/328 patients, 76.8%) according to the International Prognostic Index (IPI) and had good performance status (ECOG 0; 311/328 patients, 94.8%). Serum lactate dehydrogenase (LDH) levels were primarily in the normal range (280/328 patients, 85.4%). B symptoms (> 10% weight loss in 3 months, night sweats, and fever) were seen in 14% of the patients (Table 1).
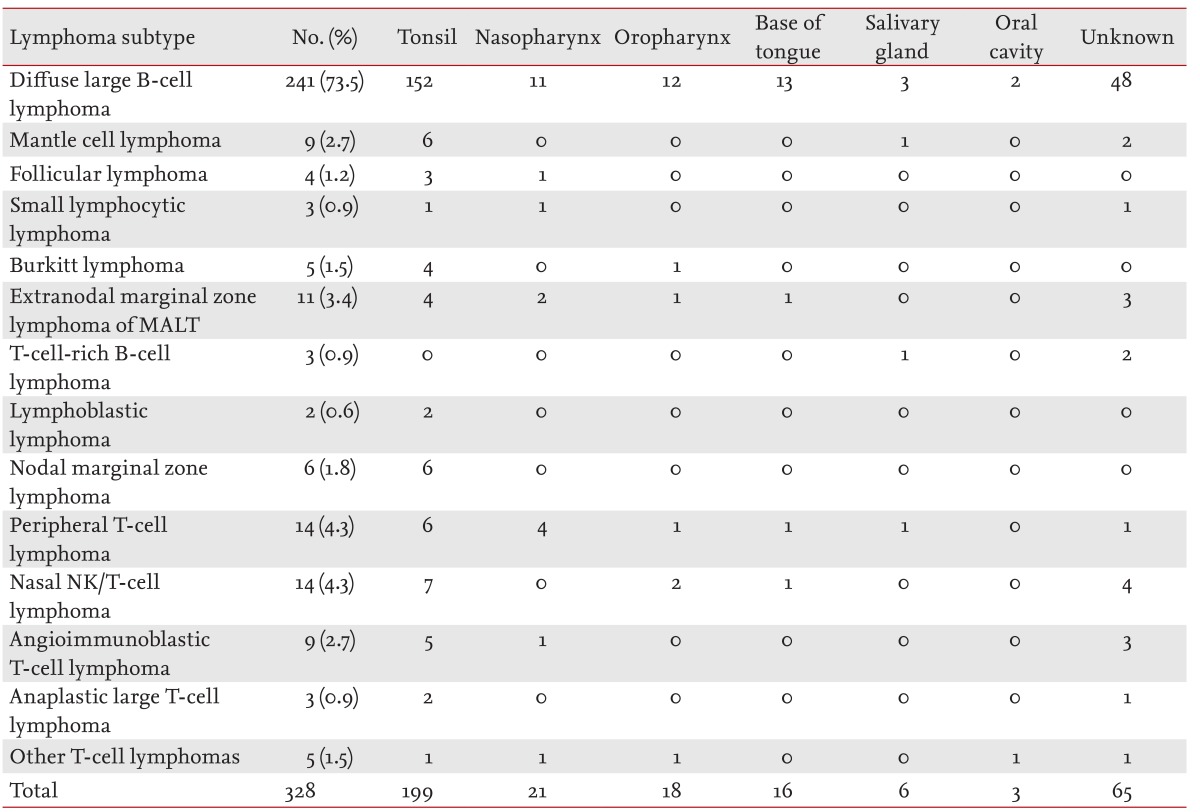
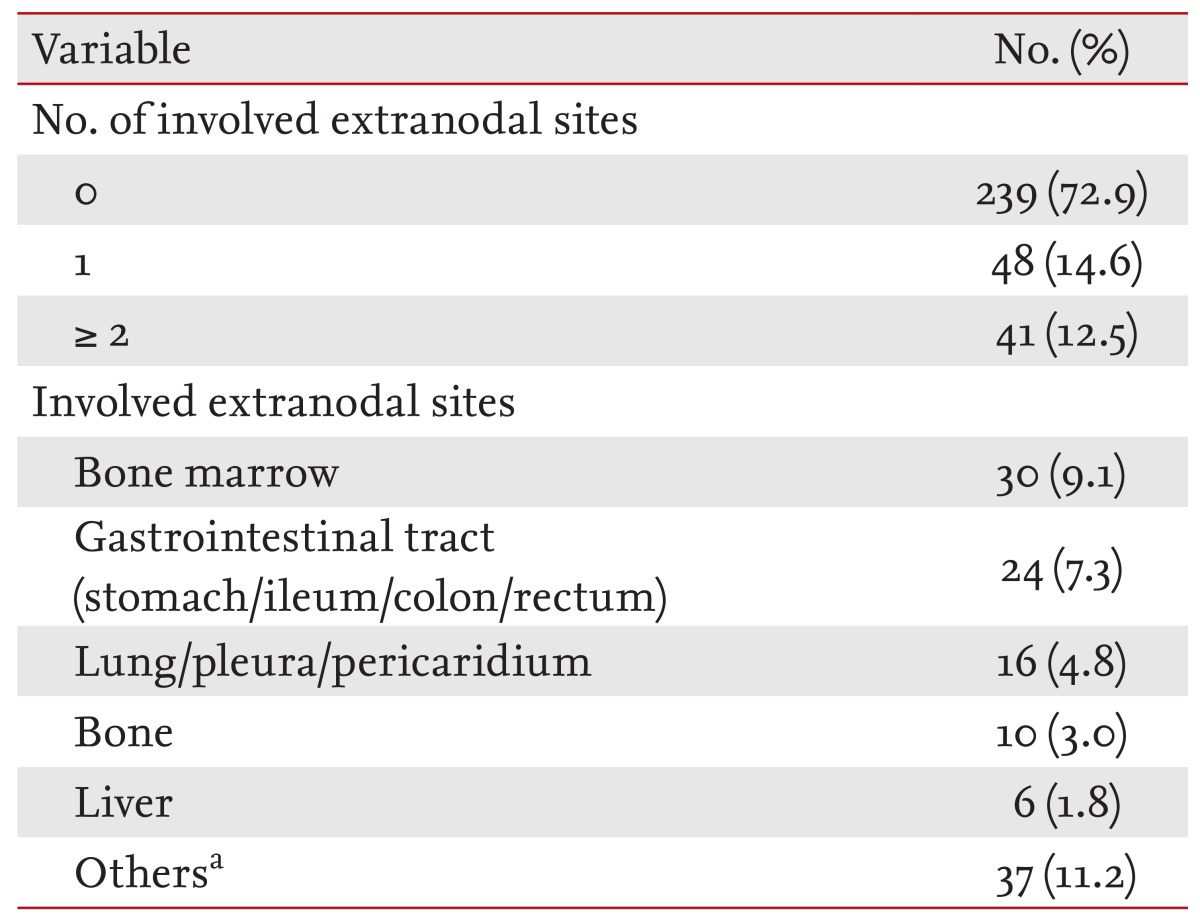
The tonsils (60.6%) were the most common site of involvement in WR-NHL, followed by the nasopharynx (6.4%), oropharynx (5.4%), sublingual site (4.8%), and salivary glands (1.8%). Of the cases with tonsillar involvement, 15% (30 cases) also had involvement of the nasopharynx or oropharynx (Table 2).
B-cell lineage NHL (281 patients, 85.6%), compared with T-cell lineage NHL, was predominant among WR-NHL cases. Diffuse large B-cell lymphoma (DLBCL; 241/281 patients, 85.8%) was the most commonly observed subtype of B-cell lineage lymphoma. Peripheral T-cell lymphoma (14 patients, 4.3%), and NK/T-cell lymphoma (14 patients, 4.3%) were the most common subtypes of T-cell lineage WR-NHL. Other subtypes included extranodal marginal zone B-cell lymphoma (11 patients, 3.4%), mantle cell lymphoma (nine patients, 2.7%), and follicular lymphoma (four patients, 1.2%). Other subtypes of NHL, excluding DLBCL, showed low prevalence (< 5%).
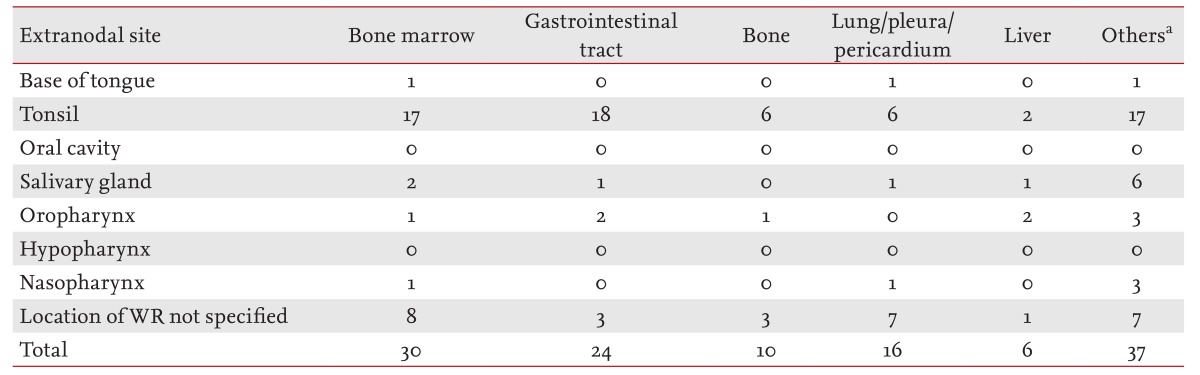
Extranodal NHL was observed in 89 patients (27.1%). Bone marrow was the most common site of involvement (n = 30), followed by the gastrointestinal tract (n = 24); lung, pleura, and mediastinum (n = 16); bone (n = 10); and liver (n = 6). A small number of cases also involved the kidneys, ovaries, testis, cerebrospinal fluid, skin, nasal cavity, brain parenchyma, eyelid, conjunctiva, trachea, glottis or the thyroid gland. A total of 48 patients (14.6%) had one extranodal site of involvement, while 41 patients (12.5%) had more than one (Tables 3 and 4). Of the 281 patients with B-cell lineage lymphoma, extranodal involvement was observed in 69 patients (24.5%). Of the 47 patients with T-cell lineage lymphoma, extranodal involvement was observed in 20 patients (42.5%); furthermore, a higher incidence was found in patients with nasopharyngeal involvement. In patients with DLBCL, the most common site of involvement was the gastrointestinal tract. Of the nine patients with mantle cell lymphoma, six had involvement of the bone marrow.
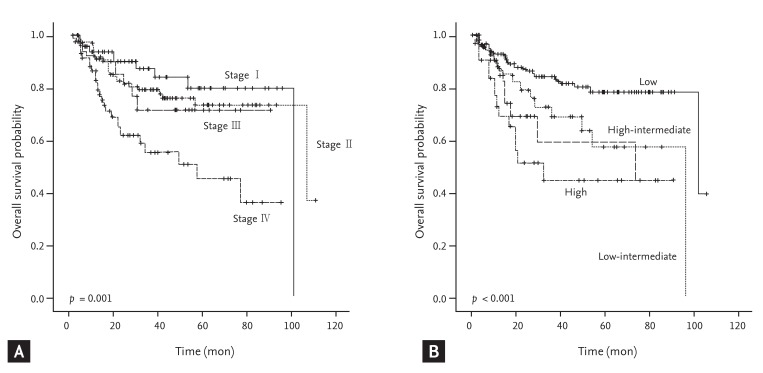
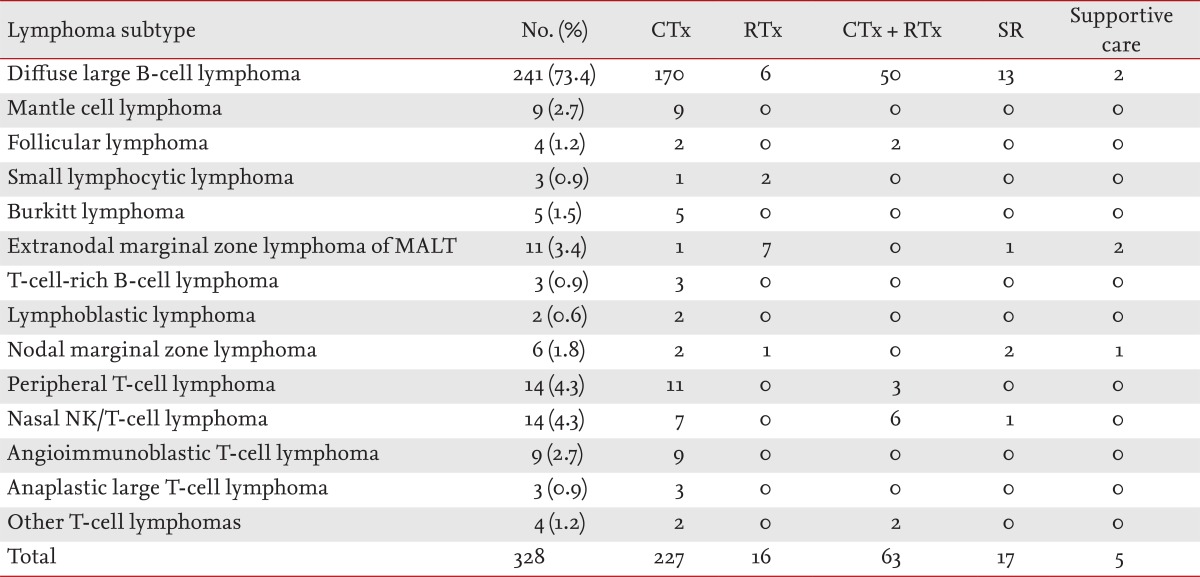
Patients with WR-NHL showed distinct differences in OS according to stage or IPI (Fig. 1). There were 227 patients in the chemotherapy alone group (69.2%), 63 patients in the chemotherapy plus radiotherapy group (19.2%), 16 patients in the radiotherapy alone group (4.9%), 17 patients in the surgical resection group (5.2%), and five patients in the supportive care group (1.5%). Most of the patients in the surgical resection group had DLBCL, and all received tonsillectomy. Of the surgical resection group, nine patients received chemotherapy, two received radiation therapy, and five received combined chemoradiotherapy after tonsillectomy. One patient did not receive any additional treatment after tonsillectomy (Table 5).
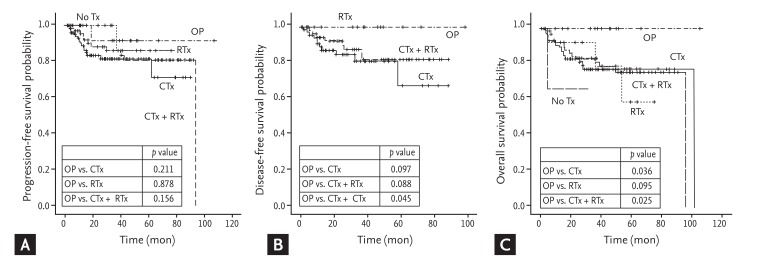
The median follow-up duration was 24.2 months (range, 0.2 to 106.1 months). The surgical resection group tended to have marginally better DFS compared with the chemotherapy alone group (2-year DFS rate, 100% vs. 84.6% ┬▒ 4.8%; p = 0.097) or chemotherapy plus radiotherapy group (2-year DFS rate, 100% vs. 92.1% ┬▒ 3.8%; p = 0.088). The surgical resection group also showed improved OS compared with the chemotherapy alone group (2-year OS rate, 100% vs. 83.1% ┬▒ 4.2%; p = 0.036) or the chemotherapy plus radiotherapy group (2-year OS rate, 100% vs. 82.9% ┬▒ 5.0%; p = 0.025) (Fig. 2).
In this study, patients aged Ōēź 62 years were found to have a lower OS rate. In addition, poor performance status (ECOG > 1), B symptoms, elevated serum LDH level, HBsAg (+), hepatitis C virus (HCV) Ab (+), Epstein-Barr virus (EBV) (+), extranodal site number > 1, T-cell subtype, high-intermediate/high risk IPI, advanced stage (Ann Arbor stage III/IV), and no CR after first-line treatment were found to be significantly poor prognostic factors by univariate analysis (Table 6). Among these factors, age Ōēź 62 years, T-cell subtype, and no CR after first-line treatment were verified to be significantly poor prognostic factors by multivariate analysis (Table 7).
Pathological distributions and baseline characteristics of WR-NHL patients in Korea were analyzed. Similar to previous studies, the most frequent pathological subtype was DLBCL [9,13,14]. While in general the DLBCL subtype involves the bone marrow in 10% to 15% of cases, the DLBCL subtype involving the bone marrow comprises only 3.3% of WR-NHL cases; furthermore, relatively few of these cases have any extranodal involvement [5,7,9].
NK/T-cell lymphoma and peripheral T-cell lymphoma occurred more frequently in this study than has been reported from Western nations. Our data showed a low incidence of follicular lymphoma (1.2%); however, it is the second most common lymphoma subtype of WR-NHL in Western populations [15]. Our results were similar to those reported in Japanese and Chinese studies [7,15,16,17]. The distribution of histological subtypes depends on regional and racial differences; therefore, this distribution might be related to susceptibility of the host to the EBV and human T-cell leukemia virus [13,18,19,20]. We also performed serum immunological studies of EBV infection in some patients (n = 79). Of 17 patients positive for EBV antibodies, five had T-cell lineage WR-NHL. EBV infection did not affect the survival outcomes; however, further evaluation to confirm the relationship between EBV infection and T-cell lineage WR-NHL may be helpful to determine the etiology of WR-NHL.
DLBCL has a heterogeneous nature, and it varies in its response to treatment as well as in its prognosis [15,21,22,23]. While it was reported that the germinal center B-cell-like (GCB) type has a better response to treatment, DLBCL with BCL2 rearrangement showed a poor response to treatment [24,25,26,27]. In this study, tissues from the WR of 56 patients were subjected to immunohistochemical evaluation of markers such as CD10, BCL6 and MUM1. These patients were divided into GCB and non-GCB groups according to the Han's criteria [27], and the differences in survival between the two groups were determined. There was no statistically significant difference between the groups, which could be due to evaluation of an insufficient number of patients to confirm the immunohistochemical staining results.
In the current study, among those with localized disease, the surgical resection group showed improved survival compared with the chemotherapy or chemotherapy plus radiotherapy group. Almost all patients in the surgical resection group had the DLBCL subtype and underwent tonsillectomy for confirmation of the pathological diagnosis. Therefore, it is possible that the surgical resection group had better survival, because tonsillar involvement itself may be a favorable prognostic factor compared with involvement of other sites. Mohammadianpanah et al. [28] reported that tonsillar lymphoma tended to be localized and to have a good outcome. They showed that combined chemotherapy and radiation therapy was highly effective and probably curative for the majority of patients with stage I, nonbulky disease [28]. Our data also showed relatively good outcomes in the chemotherapy and the chemotherapy plus radiotherapy group. However, all patients in the surgical resection group survived, and all but one received chemotherapy or chemoradiotherapy after surgery. Although a small number of patients were analyzed in our study, it is speculated that in patients with localized DLBCL, surgical resection followed by chemotherapy or chemoradiotherapy might be a better treatment than other modalities.
Prognostic factors related to survival were subjected to univariate and multivariate analyses. The T-cell lineage of WR-NHL was a significant poor prognostic factor. These findings could be related to the greater extranodal involvement, especially in the bone marrow and liver, at the time of diagnosis in these patients, compared with those with the B-cell subtype. Involvement of remote lymph nodes or extranodal involvement was found in the majority of T-cell lineage NHL cases. Conversely, most B-cell lineage NHL cases presented with spreading to adjacent lymph nodes [9,17]. The IPI and Ann Arbor stage are commonly used to assess prognosis in WR-NHL. Age Ōēź 62 years, elevated LDH levels, and advanced stages were significant poor prognostic factors for survival [5,14,17]. Failure to achieve CR after first-line treatment was also a significant poor prognostic factor according to the multivariate analysis.
In conclusion, DLBCL was found to be more frequent in Korea than reported in WR-NHL studies from other nations. And T-cell lineage NHL was found to occur more frequently than follicular lymphoma. T-cell lineage NHL, age Ōēź 62 years, and failure to achieve CR after first-line treatment were all significant poor prognostic factors for overall survival according to the multivariate analysis.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893ŌĆō2917PMID : 21351269.


2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74ŌĆō108PMID : 15761078.


4. Weisenburger DD. Epidemiology of non-Hodgkin's lymphoma: recent findings regarding an emerging epidemic. Ann Oncol 1994;5(Suppl 1):19ŌĆō24PMID : 8172811.

5. Hoppe RT, Burke JS, Glatstein E, Kaplan HS. Non-Hodgkin's lymphoma: involvement of Waldeyer's ring. Cancer 1978;42:1096ŌĆō1104PMID : 100200.


7. Yong W, Zhang Y, Zheng W, Wei Y. Prognostic factors and therapeutic efficacy of combined radio-chemotherapy in Waldeyer's ring non-Hodgkin lymphoma. Chin Med J (Engl) 2000;113:148ŌĆō150PMID : 11775540.

8. Jacobs C, Weiss L, Hoppe RT. The management of extranodal head and neck lymphomas. Arch Otolaryngol Head Neck Surg 1986;112:654ŌĆō658PMID : 3516178.


9. Ko YH, Lee JD, Kim CM, Kim IS, Lee MJ. Malignant lymphomas of the nasal cavity and Waldeyer's ring: clinicopathologic and immunohistochemical study. J Korean Med Sci 1992;7:314ŌĆō324PMID : 1299234.



10. Yamanaka N, Harabuchi Y, Sambe S, et al. Non-Hodgkin's lymphoma of Waldeyer's ring and nasal cavity: clinical and immunologic aspects. Cancer 1985;56:768ŌĆō776PMID : 4016670.


11. In: Swerdlow SH, Campo E, Harris NL, eds, et al. WHO Classification: Pathology and Genetics of Tumors of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: International Agency for Research on Cancer, 2008.
12. In: Jaffe ES, Harris NL, Stein H, eds, et al. WHO Classification: Pathology and Genetics of Tumors of Haematopoietic and Lymphoid Tissues. 3rd ed. Lyon: Agency for Research on Cancer, 2001.
13. Wang SS, Cozen W, Cerhan JR, et al. Immune mechanisms in non-Hodgkin lymphoma: joint effects of the TNF G308A and IL10 T3575A polymorphisms with non-Hodgkin lymphoma risk factors. Cancer Res 2007;67:5042ŌĆō5054PMID : 17510437.


14. Ryu MH, Kim BS, Kim TW, et al. Subclassification of diffuse large B-cell lymphomas according to the real classification: distinction of immunoblastic and non-immunoblastic subtypes. Korean J Med 2003;65:71ŌĆō80.
15. Krol AD, Le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, Noorduk EM. Waldeyer's ring lymphomas: a clinical study from the Comprehensive Cancer Center West population based NHL registry. Leuk Lymphoma 2001;42:1005ŌĆō1013PMID : 11697617.


16. Kwong YL, Anderson BO, Advani R, et al. Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 2009;10:1093ŌĆō1101PMID : 19880063.


17. Harabuchi Y, Tsubota H, Ohguro S, et al. Prognostic factors and treatment outcome in non-Hodgkin's lymphoma of Waldeyer's ring. Acta Oncol 1997;36:413ŌĆō420PMID : 9247103.


18. Chadburn A, Hyjek E, Mathew S, Cesarman E, Said J, Knowles DM. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am J Surg Pathol 2004;28:1401ŌĆō1416PMID : 15489644.


19. Matsuoka M. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene 2003;22:5131ŌĆō5140PMID : 12910250.


20. Engels EA, Cerhan JR, Linet MS, et al. Immune-related conditions and immune-modulating medications as risk factors for non-Hodgkin's lymphoma: a case-control study. Am J Epidemiol 2005;162:1153ŌĆō1161PMID : 16251389.


21. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993;329:987ŌĆō994PMID : 8141877.


22. L├│pez-Guillermo A, Colomo L, Jimenez M, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol 2005;23:2797ŌĆō2804PMID : 15728226.


23. Ezzat AA, Ibrahim EM, El Weshi AN, et al. Localized non-Hodgkin's lymphoma of Waldeyer's ring: clinical features, management, and prognosis of 130 adult patients. Head Neck 2001;23:547ŌĆō558PMID : 11400243.


24. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937ŌĆō1947PMID : 12075054.


25. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503ŌĆō511PMID : 10676951.


26. de Leval L, Bonnet C, Copie-Bergman C, et al. Diffuse large B-cell lymphoma of Waldeyer's ring has distinct clinicopathologic features: a GELA study. Ann Oncol 2012;23:3143ŌĆō3151PMID : 22700993.


Figure┬Ā1
Overall survival of Waldeyer's ring non-Hodgkin lymphoma according to stage (A) and International Prognostic Index (B).
Figure┬Ā2
Progression-free survival (A), disease-free survival (B), and overall survival (C) of Waldeyer's ring non-Hodgkin lymphoma with localized disease according to treatment. CTx, chemotherapy; RTx, radiotherapy; OP, surgical resection +/- chemotherapy or radiotherapy.
Table┬Ā6
Univariate analysis of prognostic factors for patients with Waldeyer's ring non-Hodgkin lymphoma





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



