 |
 |
| Korean J Intern Med > Volume 28(5); 2013 > Article |
|
Abstract
Chemotherapy is indispensable for the treatment of advanced biliary tract cancer. Recently, reports regarding first-line chemotherapy have increased, and first-line chemotherapy treatment has become gradually more sophisticated. Gemcitabine and cisplatin combination therapy (or gemcitabine and oxaliplatin combination therapy) have become the standard of care for advanced biliary tract cancer. Oral fluoropyrimidines have also been shown to have good antitumor effects. Gemcitabine, platinum compounds, and oral fluoropyrimidines are now considered key drugs for the treatment of advanced biliary tract cancer. Several clinical trials using molecular targeted agents are also ongoing. Combination therapy using cytotoxic agents and molecular-targeted agents has been evaluated widely. However, reports regarding second-line chemotherapy remain limited, and it has not yet been clarified whether second-line chemotherapy can improve the prognosis of advanced biliary tract cancer. Thus, there is an urgent need to establish second-line standard chemotherapy treatment for advanced biliary tract cancer. Several problems exist when assessing the results of previous reports concerning advanced biliary tract cancer. In the present review, the current status of the treatment of advanced biliary tract cancer is summarized, and several associated problems are indicated. These problems should be solved to achieve more sophisticated treatment of advanced biliary tract cancer.
Biliary tract cancer (BTC) includes intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gallbladder cancer, and ampullary carcinoma. The incidence rates of BTC are high in Asia, Latin America, and eastern European countries, while the rates are relatively low in the United States and some western European countries [1]. Approximately 18,000 patients in Japan and 3,200 patients in the United States have died from this life-threatening disease [2,3]. In Korea, approximately 5,400 new patients have been diagnosed, and approximately 3,800 patients have died from BTC [4]. Although the incidence of extrahepatic cholangiocarcinoma has remained constant, the incidence of intrahepatic cholangiocarcinoma has increased recently [3,5].
Curative resection has been considered the only chance to cure this life-threatening disease. Only 10% of patients present with early stage disease and are considered surgical candidates in Western countries [6]. However, curative resection rates have been reported to be 68.1% in cholangiocarcinoma, 68.7% in gallbladder cancer, and 93.0% in ampullary carcinoma from a Japanese registry [7]. The resection rate for BTC differs greatly between Japan and Western countries, a finding that might affect the prognosis of unresectable BTC. However, even patients who are treated with surgery ultimately experience recurrence. Moreover, many patients are still diagnosed at an advanced stage and are not candidates for surgery. Furthermore, there are many elderly BTC patients whose disease is sometimes considered unresectable because of comorbidities. Thus, chemotherapy is indispensable for the treatment of advanced BTC.
Recently, reports concerning chemotherapy for advanced BTC in the first-line setting have increased, and the first-line chemotherapy treatment has gradually become more sophisticated. Additionally, the prognosis of advanced BTC has improved, and the median overall survival has reached almost 1 year [8,9]. However, only limited studies have been reported regarding second-line chemotherapy, and it has not been demonstrated whether second-line chemotherapy can actually improve the prognosis of advanced BTC.
Despite progress in chemotherapy for advanced BTC, several problems have persisted. No consensus regarding surgical indications has been established. Not only cases involving each biliary site but also unresectable and recurrent cases have often all been included in the same study. Assessing these problems and exploring solutions to improve the quality of future evidence for advanced BTC are necessary. Thus, in this review, we summarize the current status of chemotherapy and problems associated with the treatment of advanced BTC. Additionally, we discuss strategies to improve the treatment of this disease.
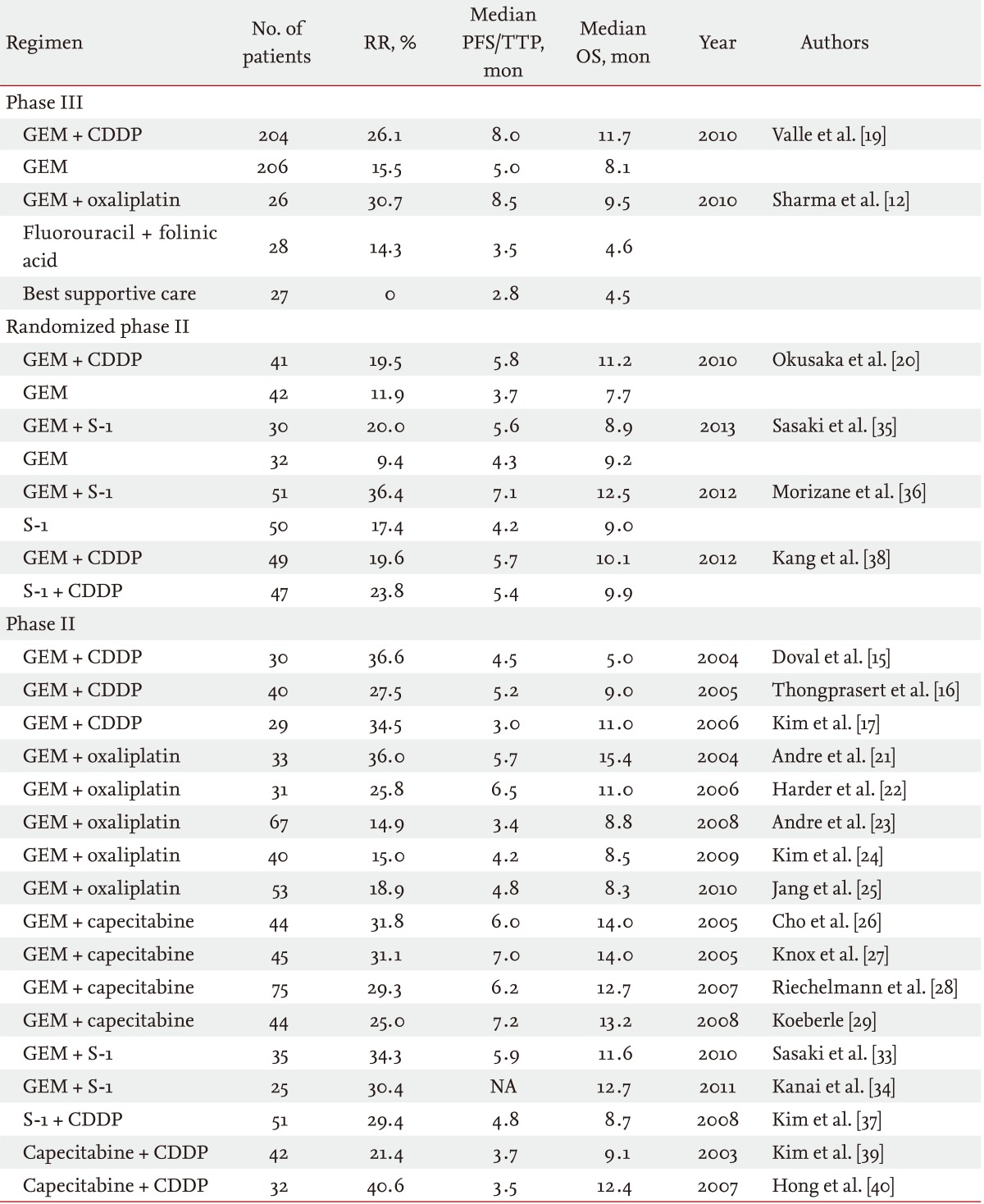
A survival benefit of chemotherapy was suggested in patients with advanced BTC in the first-line setting, based on small randomized controlled studies comparing chemotherapy with best supportive care [10-12]. Subsequently, large retrospective studies were performed, and gemcitabine and platinum were identified as promising agents for the treatment of advanced BTC [13,14]. Several phase II studies of gemcitabine and cisplatin (GC) combination therapy showed a good anti-tumor effect: the median progression-free survival and overall survival were 3.0 to 5.2 months and 5.0 to 11.0 months, respectively (Table 1) [15-17]. Based on these previous analyses, large randomized controlled studies were conducted in the United Kingdom [18,19]. In one randomized phase III study that enrolled 410 patients, the median overall survival with GC combination therapy was significantly improved compared with gemcitabine monotherapy (11.7 months vs. 8.1 months, p < 0.001). The superiority of GC combination therapy to gemcitabine monotherapy was also confirmed by a randomized phase II study (the BT-22 study) conducted in Japan [20]. According to these results, GC combination therapy became the standard of care in patients with advanced BTC in the first-line setting.
Other cytotoxic agents have also been evaluated for first-line chemotherapy in patients with advanced BTC. Gemcitabine and oxaliplatin (GEMOX) has been evaluated widely as an alternative to GC combination therapy [21-25]. The median progression-free survival and overall survival of GEMOX combination therapy were 3.4 to 6.5 months and 8.3 to 15.4 months, respectively. Oral fluoropyrimidines are considered to be the most promising agents, other than platinum compounds. Several previous studies of gemcitabine and capecitabine (GemCap) combination therapy have been conducted [26-29]. The median progression-free survival and overall survival of GemCap combination therapy were 6.0 to 7.2 months and 12.7 to 14.0 months, respectively. According to these promising data, a large randomized phase III study comparing GC combination therapy with GemCap combination therapy was conducted in Canada (ClinicalTrials.gov number, NCT00658593). Unfortunately, this randomized phase III study was stopped in December 2012 due to poor accrual. In Asian countries, particularly Japan, S-1 is widely used for BTC. Several phase II studies using S-1 monotherapy were reported [30-32]. Phase II studies of gemcitabine and S-1 (GS) combination therapy showed a good antitumor effect: the median progression-free survival and overall survival were reported to be 5.9 months and 11.6 to 12.7 months, respectively [33,34]. Regarding GS combination therapy, several regimens (3- and 4-week regimens) were reported from Japan. In the randomized phase II study comparing GS combination therapy (4-week regimen) with gemcitabine monotherapy, GS combination therapy showed a better tumor response and a longer time to progression, but the superiority in overall survival was not sufficient to select this 4-week regimen as a candidate for a phase III study [35]. However, a randomized phase II study that compared GS combination therapy (3-week regimen) with S-1 monotherapy showed the superiority of GS combination therapy to S-1 monotherapy [36]. Based on these results, a large randomized phase III study is now planned to confirm the noninferiority of GS combination therapy using a 3-week regimen of GC combination therapy in Japan. A study of S-1 and cisplatin (SP) combination therapy was also reported from Korea [37]. A randomized phase II study of SP combination therapy versus GC combination therapy showed that both regimens had comparable efficacy with a favorable safety profile as first-line chemotherapy [38]. To confirm the efficacy of SP combination therapy, a phase III study is required. Capecitabine and cisplatin (CP) combination therapy was also evaluated in Korea, and this combination also demonstrated a promising antitumor effect in patients with advanced BTC [39,40]. CP combination therapy also needs further evaluation to confirm its efficacy for advanced BTC. Regarding irinotecan and taxanes, these drugs have only shown a modest antitumor effect in the first-line setting. Thus, they are not actively being evaluated for first-line chemotherapy [41-44].
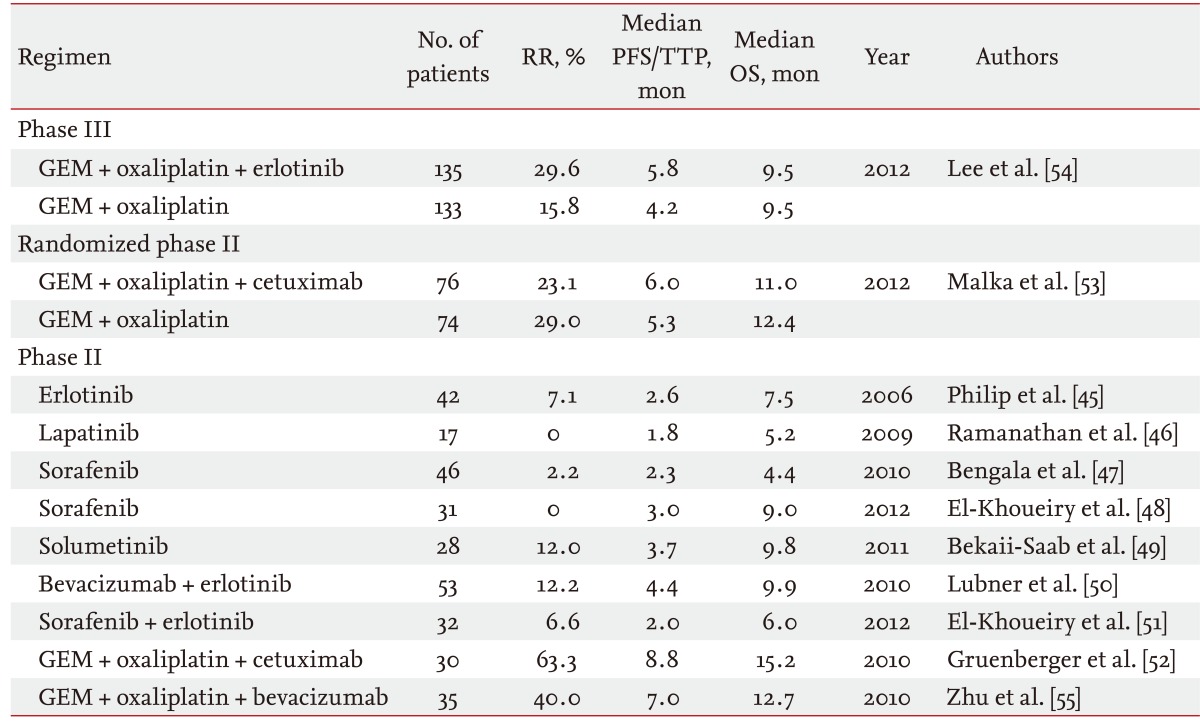
Recently, several prospective studies using molecular targeted agents for the treatment of advanced BTC have been reported (Table 2). Monotherapy using erlotinib, lapatinib, sorafenib, and selumetinib were evaluated; however, antitumor effects using these agents were extremely limited [45-49]. Combination therapy using bevacizumab and erlotinib only showed modest efficacy, with a median overall survival of 9.9 months [50]. Combination therapy using sorafenib and erlotinib also showed limited efficacy [51].
Thus, molecular-targeted agents in combination with cytotoxic agents are now the major strategy for development of new treatment regimens in this field. The combination of GEMOX with cetuximab showed an extremely good antitumor effect in a phase II study with a response rate of 53%, a median progression-free survival of 8.8 months, and a median overall survival of 15.2 months [52]. However, this combination therapy did not show significant superiority versus GEMOX alone in a phase III study, the BINGO trial [53]. Combination therapy of GEMOX with or without erlotinib was also assessed in a phase III setting, although the primary endpoint of progression-free survival was not significantly improved by adding erlotinib to GEMOX [54]. Combination therapy of GEMOX with bevacizumab showed a good antitumor effect in a phase II study, with a tumor response of 40%, a median progression-free survival of 7 months, and a median overall survival of 12.7 months [55]. A phase III study is needed to confirm the efficacy of bevacizumab.
Many prospective studies of first-line chemotherapy are ongoing for the treatment of advanced BTC. Randomized studies of chemotherapy, including phase II and phase III studies, are summarized in Table 3. Additionally, many prospective phase I or II studies, which are not listed in Table 3, are ongoing in many countries.
Few data regarding second-line chemotherapy are available for advanced BTC. No clinical study comparing chemotherapy with best supportive care exists. Moreover, the prognoses of patients treated with GC combination therapy in the ABC-02 and BT-22 studies were about the same, although the induction rates of second-line chemotherapy between these two studies were extremely different [19,20]. In the ABC-02 study, only 17.6% of the patients were treated with 5-fluorouracil-based chemotherapy. Conversely, 73.1% of the patients were treated primarily with S-1 in the BT-22 study. Thus, there was no evidence regarding whether second-line chemotherapy actually prolonged the prognosis in advanced BTC.
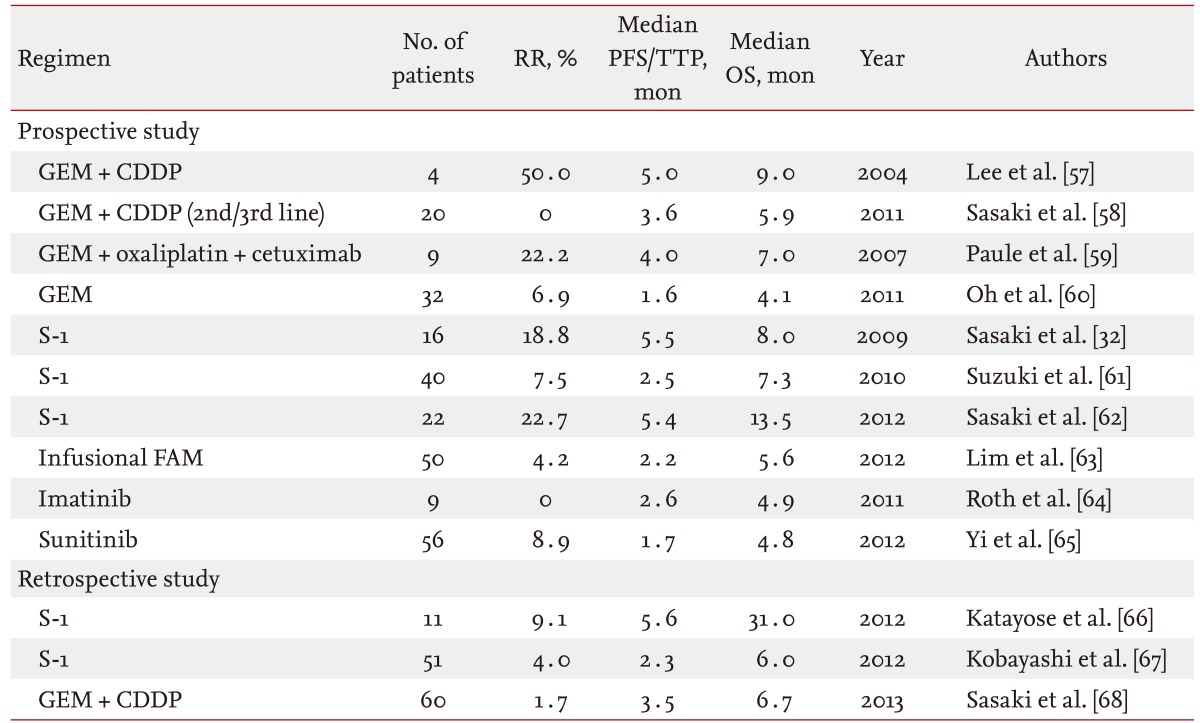
Based on a large retrospective study reported from Canada that enrolled 378 patients, only 96 patients (25%) received second-line chemotherapy [56]. In that study, many treatment regimens were evaluated together. Additionally, the objective response rate and disease control rate were 9% and 43%, respectively. The median progression-free survival and overall survival from the beginning of second-line chemotherapy were 2.8 and 7.5 months, respectively. Doublet chemotherapy and good performance status were extracted as factors associated with a better disease control rate and longer progression-free survival with second-line chemotherapy. Only a few phase II or retrospective studies of some specific regimens have been reported for advanced BTC (Table 4) [32,57-68]. Additionally, a limited number of patients were enrolled in each study, and no comparative study has been reported in the second-line setting. Further evaluation is needed to establish a standard second-line chemotherapy.
Indication criteria for surgery have not yet been defined for BTC. It may be difficult to unify the indication criteria of surgery because BTC sometimes needs extended surgery, such as hepatopancreatoduodenectomy, a highly sophisticated surgical technique. Resection rates were significantly different between Western countries and Japan [6,7]. Differences in surgical indication may lead to discrepancies in patient populations, particularly for locally advanced cases. Thus, it is necessary to create some criteria, such as the National Comprehensive Cancer Network guidelines for pancreatic cancer, criteria for resectable, borderline resectable, and unresectable disease [69]. Otherwise, it might be better to use only the data of metastatic patients in a comparative study. Moreover, the reason for unresectable disease should be indicated, in particular, whether it was caused purely by tumor factors. Factors such as patient characteristics are sometimes the cause of unresectable disease, because BTC patients are sometimes elderly and have several comorbidities.
The patient BTC population is heterogeneous. Because the incidence of BTC is low, both unresectable and recurrent cases are often enrolled in the same study. Moreover, all involved biliary sites are typically analyzed together. However, the treatment outcomes of these subpopulations are considered to differ. In fact, the median overall survivals of unresectable and recurrent patients who were treated using GC combination therapy in the BT-22 study were 9.4 and 16.1 months, respectively [20]. Furthermore, not only the antitumor effect but also the toxicity might differ between unresectable and recurrent cases, based on a pooled analysis of GS combination therapy [70]. Thus, unresectable and recurrent cases should be enrolled in different studies. Recently, adjuvant chemotherapy has been performed widely, and recurrent patients with no prior chemotherapy have decreased spontaneously.
Treatment outcomes according by biliary site were considered to be different. The prognosis of gallbladder cancer was poorer than that of other BTCs [14]. BTCs are a heterogeneous group of diseases not only anatomically but also biologically. BTCs are also a genetically diverse collection of cancers [71]. Further evaluation is needed to identify the differences in each biliary site from both basic and clinical perspectives.
Tumor volume was not assessed in most previous studies of advanced BTC. Tumor volume or tumor size is usually a prognostic factor in many other cancers. However, it is generally not discussed in the field of BTC. The patient population in BTC is usually heterogeneous. Thus, a large unresectable gallbladder cancer and a small recurrent liver metastasis are sometimes evaluated in the same patient population. Thus, we believe information regarding tumor volume should be presented together with patient characteristics. We usually use "baseline sum of the longest diameter (BSLD)" as the tumor volume parameter because BSLD is usually measured when the tumor response is judged by the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Based on our previous analysis using RECIST version 1.0, BSLD was extracted as a prognostic factor for the treatment of GS combination therapy in patients with advanced BTC [72]. Further examination is needed using a large cohort and the new RECIST criteria version 1.1.
Biliary drainage is extremely important for safe delivery of chemotherapy. The quality of biliary drainage might also affect the prognosis of advanced BTC. However, few studies provide detailed information regarding biliary drainage during the study treatment. Information concerning how many patients suspended or postponed treatment because of biliary complications is also indispensable. Thus, detailed biliary drainage information should be reported in future studies.
In the current review, we discussed the current knowledge and problems of chemotherapy for advanced BTC. The evidence for chemotherapy in advanced BTC has increased over the present decade, and the prognosis of patients with advanced BTC has improved. However, treatment regimens are not fully developed compared with other major cancers, such as those for lung cancer and colorectal cancer. Several serious problems identified in the present review remain unsolved. These problems should be addressed immediately before a large amount of evidence is accumulated in the next decade. If the incidence of BTC is too low to overcome these problems, a multinational collaboration should be considered. Global collaboration is essential for the future development of chemotherapy in the field of BTC.
References
1. Randi G, Malvezzi M, Levi F, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol 2009;20:146ŌĆō159PMID : 18667395.


2. Center for Cancer Control and Information Services, National Cancer Center. Vital statistics [Internet]. Tokyo (JP): National Cancer Center, c2010;cited 2013 Apr 18. Available from: http://ganjoho.jp/professional/statistics/statistics.html.
3. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11ŌĆō30PMID : 23335087.


4. Jung KW, Park S, Won YJ, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat 2012;44:25ŌĆō31PMID : 22500157.




5. Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 2002;37:806ŌĆō813PMID : 12445422.


6. Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist 2008;13:415ŌĆō423PMID : 18448556.


7. Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg 2009;16:1ŌĆō7PMID : 19110652.


8. Sasaki T, Isayama H, Nakai Y, et al. Improvement of prognosis for unresectable biliary tract cancer. World J Gastroenterol 2013;19:72ŌĆō77PMID : 23326165.



9. Tada M, Nakai Y, Sasaki T, et al. Recent progress and limitations of chemotherapy for pancreatic and biliary tract cancers. World J Clin Oncol 2011;2:158ŌĆō163PMID : 21611090.



10. Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593ŌĆō600PMID : 8879373.


11. Takada T, Nimura Y, Katoh H, et al. Prospective randomized trial of 5-fluorouracil, doxorubicin, and mitomycin C for non-resectable pancreatic and biliary carcinoma: multicenter randomized trial. Hepatogastroenterology 1998;45:2020ŌĆō2026PMID : 9951857.

12. Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28:4581ŌĆō4586PMID : 20855823.


13. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896ŌĆō902PMID : 17325704.



14. Yonemoto N, Furuse J, Okusaka T, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol 2007;37:843ŌĆō851PMID : 17942578.


15. Doval DC, Sekhon JS, Gupta SK, et al. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer 2004;90:1516ŌĆō1520PMID : 15083178.



16. Thongprasert S, Napapan S, Charoentum C, Moonprakan S. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol 2005;16:279ŌĆō281PMID : 15668284.


17. Kim ST, Park JO, Lee J, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 2006;106:1339ŌĆō1346PMID : 16475213.


18. Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study: The UK ABC-01 Study. Br J Cancer 2009;101:621ŌĆō627PMID : 19672264.



19. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273ŌĆō1281PMID : 20375404.


20. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469ŌĆō474PMID : 20628385.



21. Andre T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339ŌĆō1343PMID : 15319238.


22. Harder J, Riecken B, Kummer O, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer 2006;95:848ŌĆō852PMID : 16969352.



23. Andre T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 2008;99:862ŌĆō867PMID : 19238628.



24. Kim HJ, Lee NS, Lee SC, et al. A phase II study of gemcitabine in combination with oxaliplatin as first-line chemotherapy in patients with inoperable biliary tract cancer. Cancer Chemother Pharmacol 2009;64:371ŌĆō377PMID : 19142638.


25. Jang JS, Lim HY, Hwang IG, et al. Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trial. Cancer Chemother Pharmacol 2010;65:641ŌĆō647PMID : 19652971.


26. Cho JY, Paik YH, Chang YS, et al. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer 2005;104:2753ŌĆō2758PMID : 16294346.


27. Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 2005;23:2332ŌĆō2338PMID : 15800324.


28. Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 2007;110:1307ŌĆō1312PMID : 17628484.


29. Koeberle D, Saletti P, Borner M, et al. Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol 2008;26:3702ŌĆō3708PMID : 18669455.


30. Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer 2004;91:1769ŌĆō1774PMID : 15505626.



31. Furuse J, Okusaka T, Boku N, et al. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 2008;62:849ŌĆō855PMID : 18214482.


32. Sasaki T, Isayama H, Yashima Y, et al. S-1 monotherapy in patients with advanced biliary tract cancer. Oncology 2009;77:71ŌĆō74PMID : 19556812.


33. Sasaki T, Isayama H, Nakai Y, et al. Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 2010;65:1101ŌĆō1107PMID : 19707761.


34. Kanai M, Yoshimura K, Tsumura T, et al. A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 2011;67:1429ŌĆō1434PMID : 20811895.


35. Sasaki T, Isayama H, Nakai Y, et al. A randomized phase II study of gemcitabine and S-1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer. Cancer Chemother Pharmacol 2013;71:973ŌĆō979PMID : 23355041.


36. Morizane C, Okusaka T, Mizusawa J, et al. Randomized phase II trial of gemcitabine plus S-1 combination therapy versus S-1 in advanced biliary tract cancer: results of the Japan Clinical Oncology Group study (JCOG0805) [abstract]. J Clin Oncol 2012;30(4 Suppl):abstr 255.

37. Kim YJ, Im SA, Kim HG, et al. A phase II trial of S-1 and cisplatin in patients with metastatic or relapsed biliary tract cancer. Ann Oncol 2008;19:99ŌĆō103PMID : 17846018.


38. Kang MJ, Lee JL, Kim TW, et al. Randomized phase II trial of S-1 and cisplatin versus gemcitabine and cisplatin in patients with advanced biliary tract adenocarcinoma. Acta Oncol 2012;51:860ŌĆō866PMID : 22559158.


39. Kim TW, Chang HM, Kang HJ, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol 2003;14:1115ŌĆō1120PMID : 12853355.


40. Hong YS, Lee J, Lee SC, et al. Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother Pharmacol 2007;60:321ŌĆō328PMID : 17143602.


41. Sanz-Altamira PM, O'Reilly E, Stuart KE, et al. A phase II trial of irinotecan (CPT-11) for unresectable biliary tree carcinoma. Ann Oncol 2001;12:501ŌĆō504PMID : 11398883.


42. Alberts SR, Fishkin PA, Burgart LJ, et al. CPT-11 for bile-duct and gallbladder carcinoma: a phase II North Central Cancer Treatment Group (NCCTG) study. Int J Gastrointest Cancer 2002;32:107ŌĆō114PMID : 12794246.


43. Jones DV Jr, Lozano R, Hoque A, Markowitz A, Patt YZ. Phase II study of paclitaxel therapy for unresectable biliary tree carcinomas. J Clin Oncol 1996;14:2306ŌĆō2310PMID : 8708721.


44. Papakostas P, Kouroussis C, Androulakis N, et al. First-line chemotherapy with docetaxel for unresectable or metastatic carcinoma of the biliary tract: a multicentre phase II study. Eur J Cancer 2001;37:1833ŌĆō1838PMID : 11576836.


45. Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 2006;24:3069ŌĆō3074PMID : 16809731.


46. Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol 2009;64:777ŌĆō783PMID : 19169683.


47. Bengala C, Bertolini F, Malavasi N, et al. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer 2010;102:68ŌĆō72PMID : 19935794.



48. El-Khoueiry AB, Rankin CJ, Ben-Josef E, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs 2012;30:1646ŌĆō1651PMID : 21748296.



49. Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 2011;29:2357ŌĆō2363PMID : 21519026.



50. Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 2010;28:3491ŌĆō3497PMID : 20530271.



51. El-Khoueiry AB, Rankin C, Iqbal S, et al. SWOG 0941: a phase II study of sorafenib and erlotinib in patients (pts) with advanced gallbladder cancer or cholangiocarcinoma [abstract]. J Clin Oncol 2012;30(15 Suppl):abstr 4113.

52. Gruenberger B, Schueller J, Heubrandtner U, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol 2010;11:1142ŌĆō1148PMID : 21071270.


53. Malka D, Fartoux L, Rousseau V, et al. Gemcitabine and oxaliplatin (GEMOX) alone or in combination with cetuximab as first-line treatment for advanced biliary cancer: final analysis of a randomized phase II trial (BINGO) [abstract]. J Clin Oncol 2012;30(15 Suppl):abstr 4032.

54. Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012;13:181ŌĆō188PMID : 22192731.



55. Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 2010;11:48ŌĆō54PMID : 19932054.


56. Walter T, Horgan AM, McNamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 2013;49:329ŌĆō335PMID : 22947649.


57. Lee MA, Woo IS, Kang JH, Hong YS, Lee KS. Gemcitabine and cisplatin combination chemotherapy in intrahepatic cholangiocarcinoma as second-line treatment: report of four cases. Jpn J Clin Oncol 2004;34:547ŌĆō550PMID : 15466829.


58. Sasaki T, Isayama H, Nakai Y, et al. Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs 2011;29:1488ŌĆō1493PMID : 20607585.


59. Paule B, Herelle MO, Rage E, et al. Cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology 2007;72:105ŌĆō110PMID : 18025804.


60. Oh SY, Jeong CY, Hong SC, et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs 2011;29:1066ŌĆō1072PMID : 20358256.


61. Suzuki E, Ikeda M, Okusaka T, et al. A multicenter phase II of S-1 in gemcitabine-refractory biliary tract cancer [abstract]. J Clin Oncol 2010;28(15 Suppl):abstr 4145.

62. Sasaki T, Isayama H, Nakai Y, et al. Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs 2012;30:708ŌĆō713PMID : 20924641.


63. Lim KH, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Outcome of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) chemotherapy and analysis of prognostic factors in patients with refractory advanced biliary tract cancer. Oncology 2012;83:57ŌĆō66PMID : 22760079.


64. Roth A, Schleyer E, Schoppmeyer K, et al. Imatinib mesylate for palliative second-line treatment of advanced biliary tract cancer: a bicentric phase II study. Onkologie 2011;34:469ŌĆō470PMID : 21934349.


65. Yi JH, Thongprasert S, Lee J, et al. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer 2012;48:196ŌĆō201PMID : 22176869.


66. Katayose Y, Ohtsuka H, Kitamura Y, et al. An analysis of a second-line S-1 monotherapy for gemcitabine-refractory biliary tract cancer. Hepatogastroenterology 2012;59:691ŌĆō695PMID : 22469710.


67. Kobayashi S, Ueno M, Ohkawa S, et al. A retrospective study of S-1 monotherapy as second-line treatment for patients with advanced biliary tract cancer. Jpn J Clin Oncol 2012;42:800ŌĆō806PMID : 22826349.


68. Sasaki T, Isayama H, Nakai Y, et al. A retrospective study of gemcitabine and cisplatin combination therapy as second-line treatment for advanced biliary tract cancer [abstract]. J Clin Oncol 2013;31(4 Suppl):abstr 258.

69. Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2012;10:703ŌĆō713PMID : 22679115.



70. Sasaki T, Isayama H, Ito Y, et al. Comparing the treatment outcomes between unresectable and recurrent cases receiving gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer: pooled analysis of two prospective studies [abstract]. J Clin Oncol 2012;30(4 Suppl):abstr 331.

-
METRICS

- Related articles
-
Third-line docetaxel chemotherapy for recurrent and metastatic gastric cancer2013 May;28(3)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


