 |
 |
| Korean J Intern Med > Volume 28(2); 2013 > Article |
|
To the Editor,
Glomerular diseases frequently develop in patients with malignancy. It has been reported that 11% of adult patients with nephrotic syndrome also have malignant tumors [1]. Previous data show an excess incidence of de novo cancer (5.2%) after diagnosis of renal diseases in patients with biopsy proven glomerulopathy, compared to the general population [2].
Membranous glomerulonephritis (MGN) is the most common malignancy related glomerular disease [3]. Although a few reports have described membranoproliferative glomerulonephritis (MPGN) in association with lung cancer [3], MPGN related to colon cancer has to our knowledge not yet been reported. Here, we present a case of rapidly progressive MPGN associated with metastatic colon cancer.
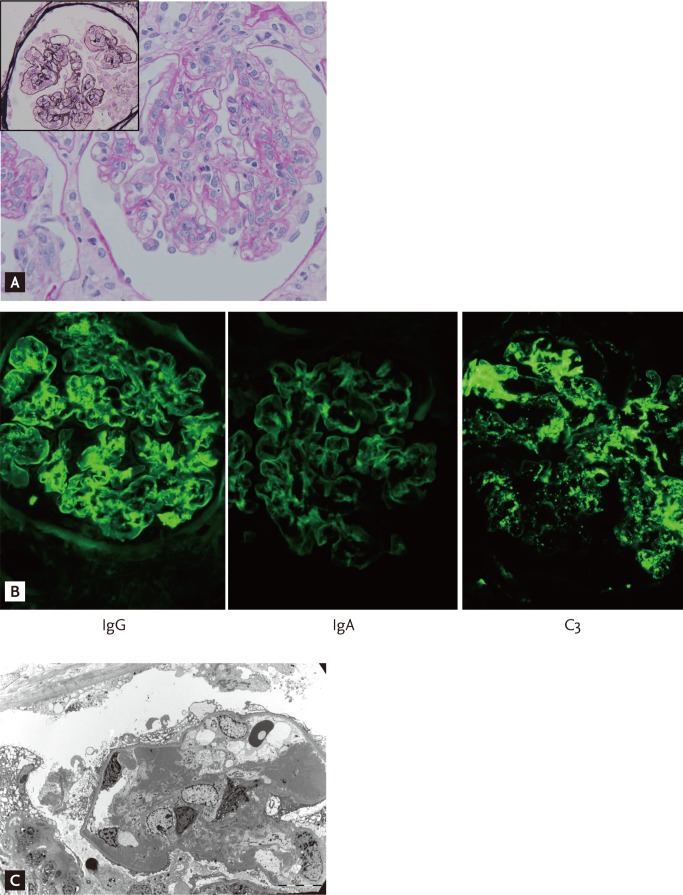
A 58-year-old male patient was admitted to our hospital for his first chemotherapy session to treat metastatic sigmoid colon cancer. The patient had been diagnosed with sigmoid colon cancer with hepatic metastasis (stage IV, T4aN1bM1) 50 days earlier. Other past medical history was unremarkable. Sigmoidectomy with partial hepatectomy had been performed 40 days earlier. The pathological type was characterized as adenocarcinoma. He was referred to the nephrology department due to abruptly increased levels of blood urea nitrogen (BUN; 68 mg/dL) and serum creatinine (sCr; 2.2 mg/dL). His laboratory results were within the normal range 50 days earlier: BUN, 11 mg/dL; sCr, 1.0 mg/dL; and there was no proteinuria or hematuria on urinalysis. On day 1 of admission, he complained of nausea, vomiting, and poor oral intake. Blood pressure was 140/70 mmHg, pulse rate was 70 beats/min, and body temperature was 37.4Ōäā. Bilateral pretibial pitting edema and moderate ascites were noted on physical examination. Laboratory studies revealed white blood cell (WBC) count 9,400/mm3, hemoglobin 10.5 g/dL, hematocrit 31.0%, platelet count 153,000/mm3, potassium 5.2 mEq/L, total protein 5.4 g/dL, albumin 2.5 g/dL, phosphorus 3.9 mg/dL, and total cholesterol 178 mg/dL. Urinalysis with microscopic examination showed urine protein (3+), a red blood cell count of 21 to 30/high power field (HPF) and a WBC count of 6 to 10/HPF. The spot urine protein/creatinine ratio was 19.9 and the fractional excretion of sodium was 0.62%. Twenty-four hour urine collection could not be performed due to a decreased urine output and poor patient cooperation. Urine protein electrophoresis showed nonselective glomerular proteinuria. Immunological studies showed no specific abnormalities. Antineutrophilic cytoplasmic antibody, antinuclear antibody, and antiglomerular basement membrane antibody were negative. Serologic markers of hepatitis B and hepatitis C were negative. The carcinoembryonic antigen level was decreased compared with the value at diagnosis (5.7 ng/mL vs. 163.9 ng/mL). Urine cytology revealed negative results. Abdominal sonography showed normal sized kidneys with appropriate echogenicity. Ascitic fluid analysis revealed transudate with no abnormal cells. On day 4 of admission, a kidney biopsy was performed because his renal function did not improve. Upon light microscopic examination, glomerular enlargement due to diffuse endocapillary proliferation, diffuse thickening of capillary loop with double contour, subendothelial and mesangial fuchsinophilic deposition, and glomerular neutrophil infiltration were found. Cellular crescents were found in four of 24 glomeruli. Mild tubular atrophy, interstitial edema, fibrosis, and mild neutrophilic and mononuclear cellular infiltrations were found. Immunofluorescence microscopy showed immunoglobulin G (IgG), IgA, C1q, dominant C3, fibrinogen, and mild IgM, as well as C4 deposition in both the mesangium and capillary loops. These findings were compatible with type 1 MPGN (Fig. 1). Chemotherapy was delayed due to the poor general condition of the patient. On day 12 of admission, hemodialysis was initiated because BUN and sCr levels were elevated to 57 and 4.2 mg/dL, respectively. His uremic symptoms, including nausea, and vomiting were aggravated, and daily urine output was < 500 mL. On day 20 of admission, the first chemotherapy session was performed with FOLFOX (oxaliplatin, leucovorin, and 5-fluorouracil). After that, four more cycles of FOLFOX chemotherapy were performed at intervals of 2 weeks. Follow-up abdominopelvic computed tomography at 3 months showed no evidence of recurrence of primary cancer. The patient is currently undergoing maintenance hemodialysis three times per week.
Various glomerular diseases such as MGN and minimal change disease frequently present as a paraneoplastic syndrome in different solid or hematological malignancies. Although there are some case reports of MPGN associated lung cancer or renal cell carcinoma [3], MPGN associated solid organ malignancy has rarely been reported.
The mechanisms that underlie how malignancy induces renal diseases remain unresolved. Three hypotheses have been suggested to explain the pathogenesis of tumor associated glomerulonephritis: 1) an undiagnosed malignancy associated with antigen deposition may have caused glomerular disease-like paraneoplastic syndrome; 2) immunosuppressive therapy used in glomerular disease may trigger tumorigenesis; and 3) viral infection may have induced both glomerulopathy and cancer by: intrinsic viral oncogenic activity; disrupted renal clearance of biological mediators associated with oncogenesis; or both [2].
The treatment of MPGN depends in part upon the underlying cause because most cases appear to have an identifiable chronic disease with circulating immune complexes [4]. However, there are limitations in proving the effectiveness of immunosuppressive drugs in progressive MPGN. Furthermore, the development of severe nephrotic syndrome, renal insufficiency, hypertension at presentation, and negative findings of renal biopsy (formation of crescents and tubulointerstitial disease) are known as poor prognostic factors [5]. We did not administer any immunosuppressive agents to our patient because he was considered to have a low probability of renal recovery, despite treatment of MPGN itself, and he was at higher risk due to his poor general condition.
In this patient, amelioration of renal function after cancer excision and chemotherapy was not observed. Based on this finding, we cannot rule out a coincidental association of MPGN with colon cancer. However, remnant metastasis may have been present, although the macroscopic masses were removed by surgery. Nevertheless, cytotoxic therapy was not performed due to age, poor general condition, drug toxicity, and low life expectancy. Other glomerular diseases such as crescentic glomerulonephropathy or acute tubular necrosis (ATN) were possible causes of the aggravated renal function. However, the pathology showed that the crescentic lesions comprised < 50% of the total and no findings compatible with ATN were identified. If ATN was the cause of the renal dysfunction, renal function should have recovered after conservative treatment. However, his renal function remains exacerbated. These findings suggest that renal failure in this patient was caused by rapid progress of glomerulopathy associated malignancy. In addition, there were no additional etiologies to explain the development of MPGN. Therefore, we believe that colon cancer may have contributed to the development of MPGN in our patient, in part, because the development of idiopathic MPGN in a relatively elderly individual is uncommon. We report here a case of rapidly progressive MPGN associated with metastatic colon cancer.
References
1. Lee JC, Yamauchi H, Hopper J Jr. The association of cancer and the nephrotic syndrome. Ann Intern Med 1966;64:41ŌĆō51PMID : 5900782.


2. Birkeland SA, Storm HH. Glomerulonephritis and malignancy: a population-based analysis. Kidney Int 2003;63:716ŌĆō721PMID : 12631139.


3. Usalan C, Emri S. Membranoproliferative glomerulonephritis associated with small cell lung carcinoma. Int Urol Nephrol 1998;30:209ŌĆō213PMID : 9607894.


Figure┬Ā1
(A) A glomerulus showing a membranoproliferative pattern of injury. There was hypercellularity due to diffuse mesangial and endocapillary proliferation with neutrophilic infiltration (periodic acid-methenamine silver stain, ├Ś 400). Capillar y loops were thickened, and double contoured (inlet, Jones methenamine silver stain, ├Ś 400). (B) Predominant immunoglobulin G (IgG), C3, and mild IgA deposits are noted in both mesangium, and capillary loops on immunofluorescence stains. (C) Confluent electron dense deposits are found in mesangium, and subendothelial side of capillary loops on transmission electron microscopic examination (├Ś 3,000).
-
METRICS

- Related articles
-
A case of polymyositis associated with Hashimoto's thyroiditis2013 May;28(3)
A Case of Erythema Nodosum and Serositis Associated with Myelodysplastic Syndrome2005 June;20(2)
A Case of Pseudomembranous Colitis Associated with Rifampin2004 December;19(4)
A Case of Glomerulonephritis in Association with Pyogenic Liver Abscess2001 September;16(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


