INTRODUCTION
Electrocardiographic (ECG) artefacts may closely simulate both supraventricular and ventricular tachycardias [1-5]. An erroneous diagnosis of an ECG artefact as an arrhythmia can lead to unnecessary diagnostic measures and interventions [3]. It must be emphasized, however, that there are obviously dire consequences of failing to diagnose some arrhythmias; for example, ventricular tachycardia, which can be a precursor of ventricular fibrillation leading to sudden death, or even long-standing supraventricular tachycardia that may lead to heart failure. Here, we describe a case of an ECG artefact diagnosed through unnecessarily complex measures, instead of simple cardiac auscultation.
CASE REPORT
The patient was a 30-year-old female, with a past history of a traffic accident, which resulted in tetraplegia and autonomic dysreflexia as sequelae of trauma to the neck region. Since the accident, the patient had experienced repeated attacks of severe renal colic and urinary tract infections. These attacks were associated with episodes of hypertension and headache.
The patient was admitted to the intensive care unit to receive epidural anaesthesia after new repeated severe attacks of abdominal pain. During observation at the intensive care unit, the electrocardiogram monitor showed an apparent narrow QRS tachycardia with a QRS frequency of 150 to 180/min. She received repeated metoprolol injections 2 to 5 mg which had a doubtful effect. A few hours later, the apparent tachycardia worsened and the QRS frequency increased to 200 to 300/min. Her blood pressure was normal. The arrhythmia was interpreted as rapid atrial fibrillation by the managing internal medicine team, who administered intravenous sotalol 40 + 20 + 20 mg, which had no apparent effect on the arrhythmia.
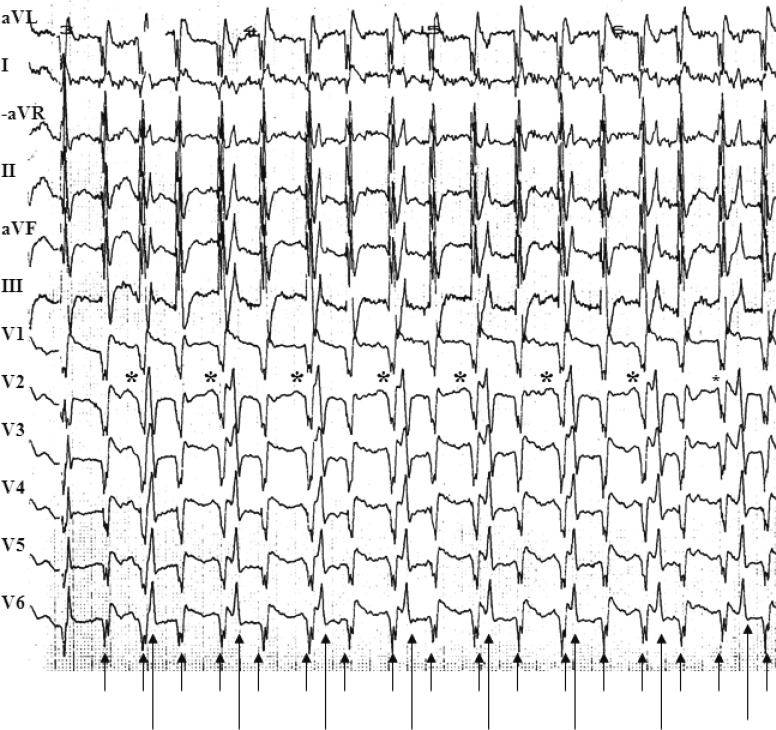
The 12-lead surface ECG showed irregular narrow QRS complex tachycardia (Fig. 1). Two rhythms with two different QRS complex morphologies were noted in the same ECG. The first rhythm was irregular and had a frequency of 250 to 300/min (the rapid rhythm). The second was regular with a frequency of 130/min (the slower rhythm). The patient was very anxious, panic-stricken, and quivering all over. Because the initial drug treatment was ineffective, and the arrhythmia was at times apparently regular, further evaluation of the case by esophageal ECG recording was performed and repeated adenosine injections were administered (Fig. 2).
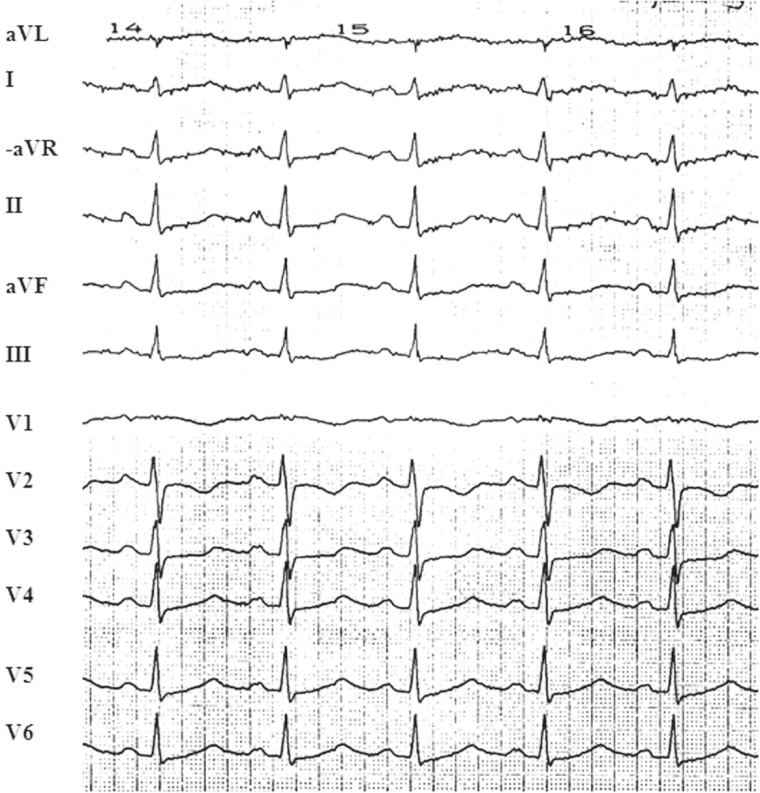
The esophageal ECG recording was made under chaotic circumstances. The patient did not like having the electrode in her esophagus. The esophageal electrode was inserted deeply in the esophagus and then pulled back very slowly while recording the ECG, including the esophageal lead, at a paper speed of 50 mm/sec and an amplification of 10 mm/mV. Adenosine 5, 10, and 15 mg were administered via the jugular vein. It was not possible to conclude what the esophageal ECG showed at the moment of examination, and the adenosine had no effect on the rapid tachycardia. A decision was made to perform electrical cardioversion. For anesthetic induction, sodium pentothal was administered. Surprisingly, the "resistant arrhythmia" terminated abruptly during sodium pentothal administration, and the ECG showed regular sinus tachycardia thereafter (Fig. 3).
Upon review of the esophageal ECG recording (Fig. 2A), P waves were noted before every QRS complex of the slower rhythm; this P wave was unrelated to the rapid rhythm. After identification of P waves in the esophageal ECG recording, a closer review of the 12-lead surface ECG (Fig. 1) also revealed P waves before every QRS complex of the slower rhythm. Injection of adenosine 10 mg intravenously via the jugular vein reduced the slower rhythm to about 45 to 50 beats/min, and adenosine 15 mg caused further slowing (total block) of the slower rhythm, with the maximum recorded R-R interval of 3.5 seconds (Fig. 2B). Even the blocked P waves can be seen clearly in leads V2 to V6 in the same figure (asterisk) continuing through the rapid rhythm. No change in the rapid rhythm was seen during or after bolus adenosine administration. We concluded that the slower rhythm was, in fact, the regular cardiac (native) rhythm and the rapid rhythm, which did not respond to antiarrhythmic drugs but disappeared completely after sodium pentothal injection, was most probably an extracardiac artefact, mimicking atrial fibrillation.
Fortunately, the purported rapid "arrhythmia" relapsed about 30 minutes after terminating the sodium pentothal. Blood pressure monitoring showed a normal, regular tracing, with a frequency that corresponded to the slower rhythm of about 130/min. Once again, the patient was shaking all over. Palpation of the radial pulse and cardiac auscultation revealed a regular rhythm with a frequency corresponding to the blood pressure tracing and the slower rhythm on the ECG monitor. The body movements and the apparent rapid arrhythmia disappeared completely after administration of 5-mg intravenous diazepam. Thus the rapid "arrhythmia" was diagnosed as an ECG artefact due to tremulous, quivering body movements.
DISCUSSION
The ECG artefact in our patient had sufficient amplitude, duration, frequency, and rhythm to simulate atrial fibrillation, especially in the limb leads. Despite the apparent QRS frequency of up to 300/min, her blood pressure was normal. This should have prompted a further clinical examination of the patient during the arrhythmia. In fact, her blood pressure monitoring showed a very clear and regular blood pressure tracing. However, the perplexing ECG findings, with two rhythms and two different QRS complex morphologies, and the resistant nature of the arrhythmia, distracted us. These thought-provoking findings misdirected our attention from simpler explanations to a more complex possible cause for the apparent arrhythmia. This consideration and the sometimes apparently regular rhythm observed chiefly in the precordial leads resulted in an aggressive evaluation of the case, performing esophageal ECG recording, administering intravenous adenosine (Fig. 2), and failing to perform a simple clinical examination of the patient during the apparent arrhythmia.
There is limited information in the literature regarding the clinical implications of misdiagnosing an ECG artefact as supraventricular or ventricular tachycardia. Knight et al. [3] reported 12 patients with ECG artefacts that were misdiagnosed as ventricular tachycardia; these resulted in medication with lidocaine in seven patients, referral or transfer for cardiac catheterization or electrophysiological tests in other patients, implantation of a pacemaker in one, and even placement of an implantable cardioverter defibrillator in one patient. Initially, the incorrectly diagnosed ECG artefact in our case led to unnecessary repeated metoprolol and sotalol administration. The esophageal ECG recording and adenosine administration were not completely unnecessary, because they did lead us to suspect the ECG artefact diagnosis; however, this occurred through a lengthy, complicated, winding path instead of stretching the hand to measure the radial pulse or looking at the whole ECG screen and blood pressure monitoring. Sodium pentothal administration was also unnecessary; however, it terminated the misdiagnosed "arrhythmia" and obviated the need for electrical cardioversion. Luckily, the apparent "arrhythmia" relapsed, confirming the diagnosis, and the patient did not receive any further intervention or treatment with stronger antiarrhythmic drugs, with all their possible consequences.
The most likely reported causes of ECG artefacts that mimic both supraventricular and ventricular tachycardia are body movements, muscular fasciculations or contractions, tremor in patients with Parkinson's disease, poor skin-electrode contact, recorder malfunctioning, and electromagnetic interference [1-3,5,6]. ECG artefacts simulating both ventricular tachycardia and atrial flutter have been produced-and reproduced-by the arm movements that occur during tooth brushing [6]. ECG inscription of diaphragmatic contractions during anesthesia has been reported [2]. The probable cause of the ECG artefact in our case was the quivering body movements, and the ECG artefact disappeared after induction of anesthesia and sedation.
Documentation of misdiagnosed ECG artefacts is largely limited to case reports. The magnitude of the problem is unclear. One report investigated physician interpretations of an ECG artefact that mimicked ventricular tachycardia. Many physicians, including cardiologists and electrophysiologists, were reported to misdiagnose ECG artefacts as ventricular tachycardia [7]. Physicians should thus include ECG-artefact in the differential 'diagnosis' of both ventricular and supraventricular arrhythmias.
Diagnosis of ECG artefacts
There are two important characteristics that differentiate an ECG artefact from a true arrhythmia. The first is clinical and includes the absence of hemodynamic deterioration during the event. The diagnosis can be confirmed by radial pulse palpation, cardiac auscultation, and blood pressure measurements. It must be noted that the artefact arrhythmias sometimes last only few seconds and it may not be possible to perform a clinical examination at that moment. This was not an excuse in our case, in which the presumed arrhythmia lasted for several hours. The second differentiating point is the ECG characteristics. It is usually possible to identify the native QRS complexes marching through the artefact. There is no relationship between the native QRS and the artefact complexes. In our case, this finding was, in retrospect, very clear on the 12-lead surface ECG (Fig. 1). It might be easier to identify native QRS complexes in ECG artefacts mimicking supraventricular arrhythmias than in those mimicking ventricular tachycardia. The superimposition of the native QRS complexes on an ECG artefact mimicking ventricular tachycardia may appear to have black notches (notches sign) [8].
ECG artefacts, especially those resembling supraventricular arrhythmia, as in our case, show two rhythms in the same ECG with two QRS complex morphologies. There are two ECG differential diagnoses for ECG artefacts. The first is ventricular tachycardia with fusion or capture beats, which usually occur when the ventricular tachycardia is slow [9]. A fusion beat is a "hybrid" QRS complex resulting from ventricular activation from two different sources. Fusion beats during wide complex tachycardia indicate the presence of A-V dissociation and are observed most frequently during relatively slow tachycardias, allowing time between ventricular tachycardia QRSs for a supraventricular beat to propagate through the normal conduction system. Capture and fusion beats during atrial fibrillation and rapid ventricular tachycardia have been reported [10]. The second ECG differential diagnosis is supraventricular tachycardia with frequently occurring ventricular extrasystoles. It is also possible for a ventricular premature beat during atrial tachycardia to produce a fusion beat. In contrast to ECG artefact rhythms, there are good relationships between the QRS complexes in the two last-mentioned ECG diagnoses.
In conclusion, Our purpose in describing this instructive case is to show the characteristic two-rhythm ECG findings of ECG artefacts on a 12-lead surface ECG. We also emphasize the importance of repeated bedside clinical examination of patients during arrhythmias. Recognition of the ECG findings and performance of a clinical examination during the arrhythmia will minimize and curtail unnecessary diagnostic and therapeutic measures.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





