 |
 |
| Korean J Intern Med > Volume 27(3); 2012 > Article |
|
Abstract
Background/Aims
The aim of this non-randomized study was to determine the role of photodynamic therapy (PDT) in a multimodal approach for the palliation of advanced esophageal carcinoma.
Methods
Twenty consecutive patients with obstructing esophageal cancer were enrolled in this study. Each subject had dysphagia, and nine could not swallow fluid. External beam radiotherapy or a self-expandable metal stent was used following PDT for dysphagia due to recurrence of the malignancy.
Results
At 4 weeks post-PDT, a significant improvement in the dysphagia score was observed in 90% of patients, from 2.75 ┬▒ 0.91 to 1.05 ┬▒ 0.83 (p < 0.05). Patients with recurrent dysphagia underwent stent insertion at an average of 63 days (range, 37 to 90). The rate of major complications was 10%. Two esophageal strictures occurred, which were treated by placement of a modified expandable stent across the stricture. The median survival in these cases was 7.0 ┬▒ 0.6 months. One patient that was treated with PDT and radiotherapy is alive and showed a complete tumor response.
Upon presentation, 40% to 50% of patients with esophageal cancer are deemed surgically unresectable [1,2]. Despite advances in both diagnosis and treatment, the 5-year survival continues to be less than 10% [3]. Patients do not develop symptoms such as dysphagia until late in the course of the disease. Because of these two facts, more than half of patients present with disease that is locally advanced or metastatic. As many patients present with advanced disease and a cure is often not possible, palliative therapy plays a critical role in the management of esophageal cancer. Advanced esophageal carcinoma is mainly associated with dysphagia. The main goal in the palliation of patients with advanced esophageal cancer is to reduce dysphagia and to maintain nutrition and occlusion of tracheoesophageal fistula so as to improve the quality of life. Surgical esophagectomy, while an effective means of palliating dysphagia, is accompanied by marked morbidity and mortality. Multimodality therapy, consisting of dilation [4], external beam irradiation [5], brachytherapy, argon plasma coagulation [6], esophageal prosthesis [7], chemotherapy [8], and photodynamic therapy (PDT) [9], has been used as a palliation therapy for esophageal cancer. Recently, PDT has emerged as a safe and effective technique for treating esophageal cancer. PDT is a minimally invasive, organ-preserving treatment modality that causes few side effects. Early results of PDT in esophageal cancer are promising and PDT may help to lessen dysphagia, the symptom with the greatest impact on the quality of life of patients with an incurable disease [10,11].
The aim of this non-randomized study was to determine the role of PDT as an adjuvant therapy for the palliation of advanced esophageal cancer.
From January 2001 through January 2003, 20 patients with dysphagia caused by biopsy-proven esophageal cancer were enrolled in this study. None were eligible for surgical resection due to tumor involvement into the adjacent tissue, distant lymph node metastasis, poor performance status plus inoperable status due to co-morbidity, refusal of surgical intervention, or a combination of these reasons.
Clinical staging was performed by acquiring a detailed history and conducting a physical examination, barium esophagogram, esophago-gastroscopy with biopsy, abdominal ultrasonography, computed tomography (CT) scans of the chest and abdomen, and endoscopic ultrasonography.
Written informed consent was obtained from all patients undergoing PDT. This prospective study was approved by the ethics committee of Soonchunhyang University, Seoul, Korea.
The patients received 2 mg/kg porfimer sodium (Photofrin II, Axcan Pharma Inc., Mont Saint Hilaire, QC, Canada), administered intravenously over 3 to 5 minutes. Following administration of the photosensitizer, patients were instructed to avoid exposure to direct sunlight for the next 4 weeks. At 48 hours after photosensitization, the area of esophageal cancer was treated with red light at a wavelength of 630 nm from a continuous-wave dye laser (Ceralas PDT 633, CeramOptec, Bonn, Germany). The laser energy was delivered by a 2 cm long (range, 1 to 3) flexible optical quartz fiber with a cylindrical diffusing tip, which was inserted through the biopsy channel of the endoscope. The endoscope was withdrawn sequentially to deliver light over the entire tumor area.
The light dose was calculated as 300 J/cm of fiber with a power density of 400 to 600 mW/cm at the diffusing tip. The irradiation time varied accordingly from 8 to 10 minutes.
One patient treated with an uncovered esophageal stent prior to PDT had tumor ingrowth through the stent, as observed by endoscopy. This patient was also treated with PDT 17 days following stent insertion with a similar light dosage.
Patients were allowed to take clear liquids on day one following the initial PDT and remained on liquids until the follow-up endoscopy was performed 48 hours later. They were advanced to a soft diet 2 to 3 days after PDT treatment.
External beam RT was given to 10 patients following PDT for dysphagia due to recurrence of the malignancy. A total dose of 6,000 Gy was administered to all patients, 5 days per week, in divided doses over a period of 6 to 7 weeks. All patients received 170 to 200 Gy per fraction. The time interval between PDT and RT was, on average, 22 days (range, 2 to 44).
Data were collected prospectively and included the demographic profile, reason for treatment (obstruction or refusal of surgery or presence of multiple co-morbid factors), history of prior esophageal stent, history of prior therapy (e.g., dilation, chemotherapy, or RT), tumor characteristics (histology, tumor length, and location), amount of light delivered, length of the diffuser used, dysphagia score before treatment and at follow-up examination, dysphagia-free interval, and post-treatment complications.
The primary endpoint was the impact of treatment on dysphagia. Dysphagia was graded using the following scale: grade 1, difficulty in swallowing solid food; grade 2, difficulty in swallowing semisolid food; grade 3, difficulty in swallowing liquids; grade 4, inability to swallow anything, including saliva [10].
A change in the dysphagia score at 4 weeks after a single session of PDT was evaluated and compared with the baseline score. Improvement in dysphagia was considered to be successful if the baseline dysphagia score decreased by at least one point following treatment. The dysphagia-free interval was calculated from the date of documented dysphagia improvement until the date of documented worsening of dysphagia.
Persistent dysphagia was defined as dysphagia that continued after treatment without an improvement in swallowing. Recurrent dysphagia was defined as the redevelopment of dysphagia after an initial improvement of 30 days.
Complete response was defined as no visual or biopsy evidence of cancer, following random biopsies from the treatment area, with no evidence of cancer by CT.
Patient follow-up included history, physical examination, laboratory evaluation, and endoscopy at 48 hours, 1 week, 1 month, and then periodically every 3 months, and chest and abdominal CT every 3 months. Two days after PDT, endoscopy was repeated to visualize the remnant/residual lesion. All cases showing the presence of remnant (residual) lesion received a repeat course of PDT. Endoscopy was performed if the patient complained of dysphagia.
A recurrence of dysphagia was reported by patients or their families, and grades 2 or 3 were treated when present. Deterioration by at least one grade was necessary to implement rescue treatment. Patients with a recurrent obstructive tumor were given the option of further PDT treatment or placement of an expandable metal stent. A partially covered self-expansible metallic stent (SEMS; Shim-Hanaro stent, MI Tech Co., Seoul, Korea) was used in all patients receiving stents using Shim's technique [12].
All values were reported as the mean ┬▒ SD. Differences in dysphagia scores were analyzed using a paired Student's t test; p values of < 0.05 were considered to be statistically significant. Survival was calculated using the Kaplan-Meier method from the time of first PDT treatment until death.
From January 2002 to January 2004, 20 patients with advanced obstructing esophageal cancer were evaluated for their response to the adjuvant treatment protocol. Table 1 lists the patients' characteristics including age, sex, histology, mean tumor length, location, and staging of the esophageal cancer. At the time of admission patients complained of dysphagia on a solid diet (level 1, two patients), semisolids (level 2, five patients), or liquids (level 3, nine patients). Four patients complained of dysphagia (level 4) and were not able to handle saliva. Their ages ranged from 52 to 87 years (median, 66). Three patients required initial dilation prior to PDT. A flexible guide wire was passed through the biopsy channel of the endoscope, with careful dilation by balloon (Rigiflex, Microvasive, Boston Scientific Co., Natick, MA, USA) to at least 11 mm. Two patients had previous treatments other than PDT. In one, a self-expansible stent was placed for palliation of the malignancy; however, this patient had tumor ingrowth through the stent mesh. The other patient received a complete course of chemotherapy and RT; however, tumor recurrence occurred 2 months following treatment. Ten patients received RT followed by PDT. All patients received external beam RT. Twenty patients underwent 43 PDT sessions for obstructing esophageal cancer.
Tumors were located in the cervical (upper) esophagus (five patients), middle esophagus (five patients), and distal esophagus (ten patients). The mean tumor length was 6.0 ┬▒ 2.5 cm (range, 2 to 10).
Out of 20 patients, 5 received only PDT as the primary palliative treatment and 15 received PDT as part of a multimodal therapy along with stent placement or RT. The characteristics of the five patients who received PDT as the only palliative treatment are provided in Table 2. Two patients (1 and 4) required a second session of PDT at 13 and 28 days, respectively. The first patient had persistent dysphagia in spite of the first session of PDT. The dysphagia score decreased from grade 4 to 2 at the 30-day follow-up; repeated PDT was given on day 13. The second patient had recurrent dysphagia after an initial improvement in dysphagia score. This patient received repeat PDT at 28 days after the first session, and his dysphagia score improved from grade 4 to 1 at the 30-day follow-up.
At 4 weeks after PDT, a significant improvement was observed in dysphagia scores for 90% of the patients, from 2.75 ┬▒ 0.91 to 1.05 ┬▒ 0.83 (p < 0.05), with a dysphagia-free interval of 81 days.
One patient had an existing esophageal stent prior to PDT. The indication for PDT treatment in this patient was tumor ingrowth through the previously placed uncovered stent. This patient received PDT as an adjuvant treatment at a follow-up period of 17 days with similar light dosage. The dysphagia score improved from grade 3 to 1 in this patient at the 30-day follow-up.
We placed a stent in six patients following PDT. An expandable metal stent was placed after the initial PDT treatment; indications for adding an expandable metal stent after PDT were persistent dysphagia (n = 2) and recurrent dysphagia (n = 4). Upon endoscopy at 48-hour post-PDT, two patients with persistent dysphagia showed minimal tumor necrosis and were therefore classified as early PDT failures. When dysphagia persisted beyond 1 week post-PDT, stents were placed on days 14 and 15 post-PDT. Patients with recurrent dysphagia underwent stent insertion at 63 days on average (range, 37 to 90). Three patients required retreatment with PDT for persistent dysphagia (n = 1) or recurrent dysphagia (n = 2). As described above, two of these patients received only PDT as their palliative therapy. One other patient received a second course of PDT at 38 days after the first treatment.
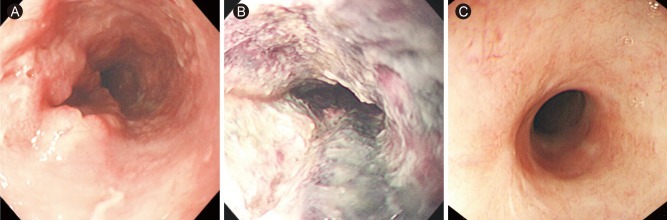
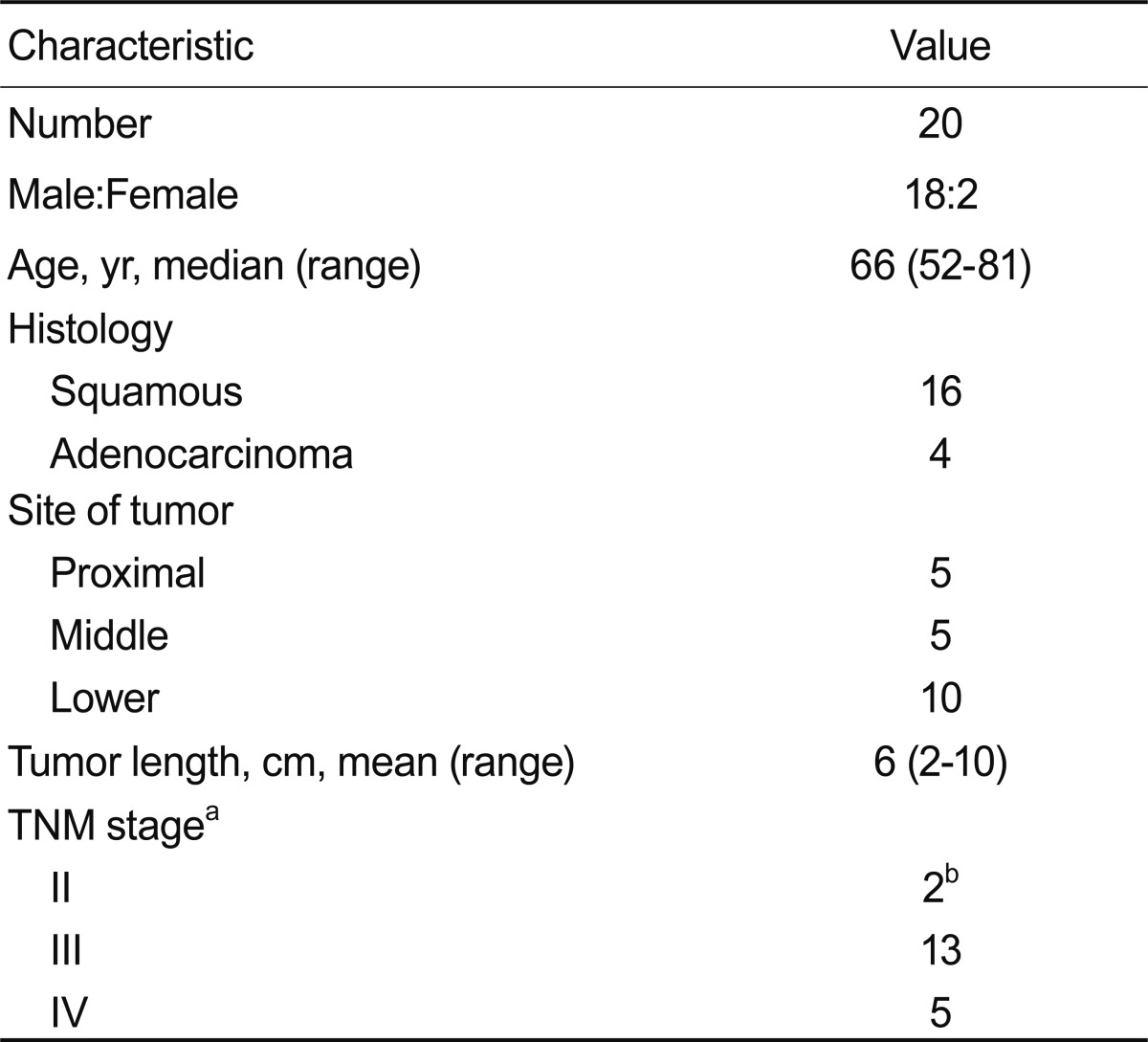
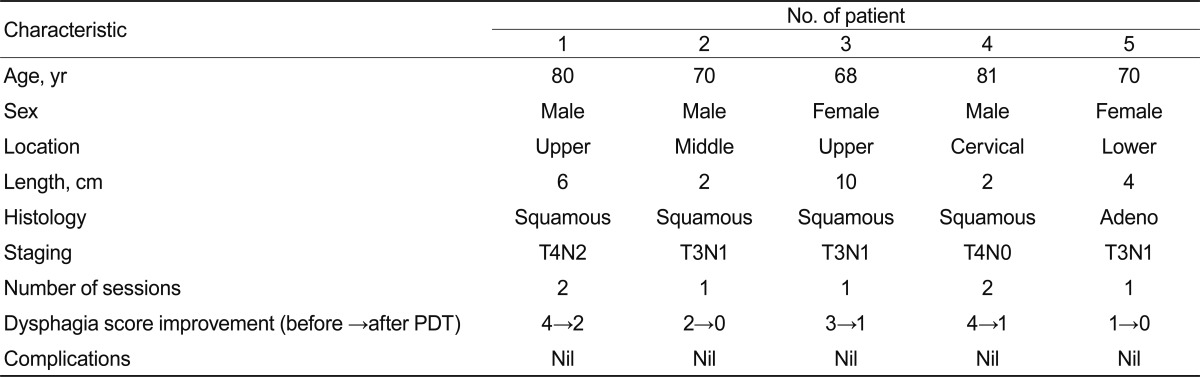
Eighteen patients (90%) died from their disease. The median overall survival among these patients was 7.0 ┬▒ 0.6 months. One patient died due to another cause. One patient, who was treated with PDT and RT, had a complete tumor response by endoscopic visual, biopsy, and CT scan (Fig. 1). This patient is still alive and has maintained a complete response for 28 months after PDT.
The intravenous administration of Photofrin was not associated with any acute toxicity. All PDT courses were performed under conscious sedation. The complications after 43 PDT courses included esophageal stricture (n = 2), skin pigmentation (n = 3), and facial edema (n = 1). Esophageal strictures following PDT were treated by placement of a modified expandable stent across the stricture. This was fixed to the esophageal mucosa by hemoclips at the upper end. After 3 months, the stent was removed in one patient and complete recannalisation of the lumen was observed.
PDT is being recognized for its use as a curative therapy in early esophageal cancer [13+15] and palliation in advanced esophageal cancer [16]. Increasingly, PDT is being used as a component of multimodality treatments for advanced esophageal cancer [16]. Palliation of dysphagia plays a central role in the management of advanced esophageal cancer. A number of therapies are available for palliation in esophageal cancer, although no single therapy has been established as being superior [17]. Choosing the method of palliation involves a detailed consideration of the risks and benefits of each method. Tumor characteristics, patient choices, physician experience, and local availability all need to be taken into consideration before making the final choice.
Surgery, chemotherapy, and RT all play a primary role in the treatment of esophageal cancer. Patients with advanced esophageal cancer are of advanced age, rather ill, and/or have multiple co-morbid factors that increase the morbidity and mortality of surgical procedures. Chemotherapy and RT also provide palliation of dysphagia; however, the relief of symptoms is delayed, multiple treatments are required, and there are considerable side effects associated with these therapies. Endoscopic palliation is gaining rapid and widespread acceptance and SEMS are used increasingly as the primary palliation in advanced esophageal cancer [7,18]. SEMS, especially in patients with advanced age or with multiple co-morbid factors, achieves rapid palliation and imparts a better quality of life. However, stent ingrowth and overgrowth can be a problem, warranting an alternative procedure. PDT is being increasingly applied for the palliation of esophageal cancer, both in isolation and as part of multimodal treatments [17,19,20]. These studies also address the role of PDT as an adjuvant treatment component in advanced esophageal cancer. PDT in isolation produced rapid and dramatic improvements in dysphagia in three patients in one session; two others required an additional session. In the remaining 15 patients, PDT was used in an adjuvant treatment in combination with stenting or RT. After 4 weeks, PDT was effective in 90% of the patients in palliating dysphagia, with a dysphagia-free interval of 81 days. Similar studies have addressed the role of PDT as part of a multimodal treatment. Luketich et al. [20] reported a study of 77 patients using PDT and stenting as a means of palliating dysphagia. PDT was effective in palliating dysphagia in 90% of patients at four weeks following PDT with a dysphagia-free interval of 80 days. Maier et al. [16] reported their experience using PDT in a multimodal treatment with RT and neodymium: yttrium-aluminum-garnet (Nd:YAG) laser. In their series of 119 patients treated with PDT and RT, PDT improved the dysphagia score in all patients with a significant improvement in tumor stenosis when PDT was followed by RT. In a multicenter study comparing PDT and Nd:YAG laser in obstructing esophageal cancer in 277 patients by Lightdale et al. [10], the relief of dysphagia was equivalent between the two groups; however, PDT had a better objective tumor response rate. Specifically, PDT required fewer procedures and showed a better response in patients who had received a prior therapy.
In patients with advanced esophageal cancer, dysphagia is the symptom with the most severe impact on quality of life. Rapid relief of dysphagia results in a better quality of life. PDT provides an effective palliation for patients with incurable disease and those who are unable to undergo surgical procedures due to multiple co-morbid factors. PDT is a non-thermal photochemical process of tumor ablation. PDT is technically easy to perform and patients have a better tolerance to the therapy, even under conscious sedation.
In this study, we used PDT in combination with SEMS and RT. PDT was used as a first-line treatment in almost all patients, except two who received CT with RT and stenting, respectively, prior to PDT and had recurrence of dysphagia. The photosensitivity caused by PDT was minor and could be managed conservatively. Esophageal stricture in two patients could be managed with SEMS.
This study demonstrates that PDT is a safe and effective component of an adjuvant treatment for obstructing esophageal cancer. With the availability of new photosensitizers that prevent skin photosensitivity and allow deeper light penetration, PDT is expected to have a place in the therapeutic armamentarium of physicians, as part of a multimodal or salvage therapy for the treatment of advanced esophageal cancer.
References
1. Sharpe DA, Moghissi K. Resectional surgery in carcinoma of the oesophagus and cardia: what influences long-term survival? Eur J Cardiothorac Surg 1996;10:359ŌĆō363PMID : 8737693.


2. Muller JM, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg 1990;77:845ŌĆō857PMID : 2203505.


4. Parker CH, Peura DA. Palliative treatment of esophageal carcinoma using esophageal dilation and prosthesis. Gastroenterol Clin North Am 1991;20:717ŌĆō729PMID : 1723968.


5. Kim CM, Hong WS, Lee JO, et al. A retrospective study on radiotherapy and radiochemotherapy in esophageal cancer. Korean J Intern Med 1988;3:58ŌĆō63PMID : 3153794.



6. Rupinski M, Zagorowicz E, Regula J, et al. Randomized comparison of three palliative regimens including brachytherapy, photodynamic therapy, and APC in patients with malignant dysphagia (CONSORT 1a) (Revised II). Am J Gastroenterol 2011;106:1612ŌĆō1620PMID : 21670770.


7. Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med 1993;329:1302ŌĆō1307PMID : 7692297.


8. Pantling AZ, Gossage JA, Mamidanna R, et al. Outcomes from chemoradiotherapy for patients with esophageal cancer. Dis Esophagus 2011;24:172ŌĆō176PMID : 21073614.


9. Yano T, Muto M, Minashi K, et al. Long-term results of salvage photodynamic therapy for patients with local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy 2011;43:657ŌĆō663PMID : 21623555.


10. Lightdale CJ, Heier SK, Marcon NE, et al. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointest Endosc 1995;42:507ŌĆō512PMID : 8674919.


11. McCaughan JS. Photody namic therapy for obstructive esophageal malignancies. Diagn Ther Endosc 1999;5:167ŌĆō174PMID : 18493499.




12. Shim CS, Cho YD, Moon JH, et al. Fixation of a modified covered esophageal stent: its clinical usefulness for preventing stent migration. Endoscopy 2001;33:843ŌĆō848PMID : 11571679.


13. Tanaka T, Matono S, Nagano T, et al. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest Endosc 2011;73:1ŌĆō6PMID : 21074765.


14. Grosjean P, Wagnieres G, Fontolliet C, van den Bergh H, Monnier P. Clinical photodynamic therapy for superficial cancer in the oesophagus and the bronchi: 514 nm compared with 630 nm light irradiation after sensitization with Photofrin II. Br J Cancer 1998;77:1989ŌĆō1995PMID : 9667680.



15. Cheon YK, Kim WJ, Cho JY, Lee JS, Lee MS, Shim CS. Outcome of photodynamic therapy for early esophageal cancer. Gut Liver 2007;1:126ŌĆō131PMID : 20485628.



16. Maier A, Tomaselli F, Gebhard F, Rehak P, Smolle J, Smolle-Juttner FM. Palliation of advanced esophageal carcinoma by photodynamic therapy and irradiation. Ann Thorac Surg 2000;69:1006ŌĆō1009PMID : 10800784.


17. Tytgat GN. Endoscopic therapy of esophageal cancer: possibilities and limitations. Endoscopy 1990;22:263ŌĆō267PMID : 1703074.


18. Ell C, May A. Self-expanding metal stents for palliation of stenosing tumors of the esophagus and cardia: a critical review. Endoscopy 1997;29:392ŌĆō398PMID : 9270922.


Figure┬Ā1
(A) Before photodynamic therapy (PDT). Endoscopy shows an ulcerofungating mass on the mid-esophagus. (B) Seven days post-PDT. Endoscopy shows coagulation necrosis with ulcer at the PDT-treated lesion. (C) Five months post-PDT. A scar is seen at the previously cancerous lesion and there is no tumor in the biopsied specimens.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



