 |
 |
| Korean J Intern Med > Volume 26(4); 2011 > Article |
|
Abstract
Background/Aims
Antiphospholipid antibodies (aPL) have been detected in various proportions of patients with primary immune thrombocytopenia (ITP), but the clinical significance of this is debatable. The present study aimed to determine the frequency and clinical implications of elevated aPL in adult patients with ITP.
Methods
We prospectively studied newly diagnosed adult patients with ITP who were enrolled between January 2003 and December 2008 at Chungnam National University Hospital. They were evaluated for the presence of lupus anticoagulant (LA) and anticardiolipin antibodies (aCL) at diagnosis and were followed for the development of thrombosis.
Results
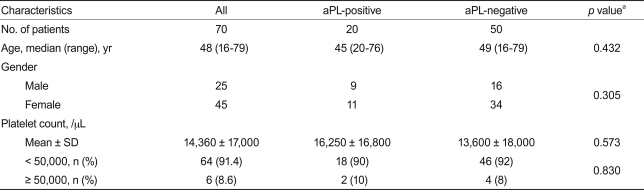
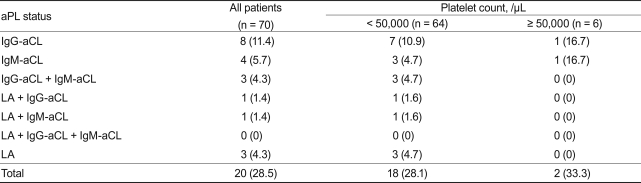
Seventy consecutive patients with ITP (median age, 48 years; range, 18 to 79) were enrolled. Twenty patients (28.5%) were positive for aPL at the time of diagnosis: aCL alone in 15 (75%), aCL and LA in two (10%), and LA alone in three (15%). Patients who had platelet counts < 50,000/µL were administered oral prednisolone with or without intravenous immune globulin. No difference was found between the aPL-positive and -negative groups regarding gender, initial platelet count, and response to the therapy. After a median follow-up of 20 months (range, 2 to 68), two of 20 patients who were aPL-positive (10%) developed thrombosis, whereas no thrombotic event was found among those who were aPL-negative.
Primary immune thrombocytopenia (ITP) is an acquired disorder characterized by isolated thrombocytopenia resulting from autoantibody-mediated peripheral platelet destruction and the absence of any obvious initiating and/or underlying cause of the thrombocytopenia [1]. Antiphospholipid syndrome (APS) is a thrombotic disorder defined by the presence of one or more clinical features of arterial or venous thrombosis, recurrent fetal loss, and presence of antiphospholipid antibodies (aPL) such as anticardiolipin antibody (aCL), lupus anticoagulant (LA), and/or anti-β2 glycoprotein-I (anti-β2GPI) [2-4]. Thrombocytopenia, as a manifestation of primary APS, has a reported prevalence of 20 to 46% [5-7]. Although evidence suggests that aPL may bind activated platelet membranes and cause platelet destruction [8,9], the pathogenesis of thrombocytopenia related to aPL remains unclear. Conversely, elevated levels of aPL have been demonstrated in patients with ITP. The reported incidences of aPL in ITP vary considerably, ranging from 26 to 75% of cases, which can be attributed partly to technical differences [5-8,10-13]. Furthermore, the clinical significance of aPL in patients who have ITP is controversial. Recently, an international working group reported that measuring aPL is not routinely recommended for investigation of ITP [1]. However, the prevalence of aPL and its clinical implications in ITP have not been studied in Korean populations. Here, we performed a prospective study to define the frequency and clinical relevance of aPL in a single-center cohort of adults with ITP.
We prospectively enrolled patients who were newly diagnosed with ITP between January 2003 and December 2008 at Chungnam National University Hospital. ITP was diagnosed based on the guidelines proposed by the American Society of Hematology [14]. Only patients aged > 18 years who had platelet counts < 100,000/µL and no history of other clinical conditions that can cause thrombocytopenia were included. All patients underwent a panel of laboratory tests, including tests for antinuclear and antivirus (cytomegalovirus, Epstein-Barr virus) antibodies, and screening for human immunodeficiency virus (HIV), and hepatitis B and C virus infection. Peripheral blood and bone marrow smears were examined to exclude other causes of thrombocytopenia. Patients who had a history of arterial or venous thrombosis were excluded. Additional exclusion criteria were a history or clinical findings of APS fulfilling the international consensus statement criteria [3], systemic lupus erythematosus (SLE) satisfying the American College of Rheumatology criteria [15,16], other autoimmune disorders, malignancies, and concomitant viral infections, including HIV or hepatitis C or B virus infection.
Blood samples were collected in vacuum tubes containing sodium citrate. Dilute Russell's viper venom time (dRVVT) was used as an initial sensitive screening test for LA and as a confirmatory test. Criteria for positive LA were based on the guidelines of the Scientific Subcommittee of the International Society on Thrombosis and Haemostasis [17]. Patients in whom a ratio > 1.2 (test dRVVT/control dRVVT) was obtained were considered to be positive.
Blood samples were collected in serum tubes. IgM-aCL and IgG-aCL tests were performed using an enzyme-linked immunosorbent assay (ELISA) for semiquantitative detection in human sera. The values of aCL are expressed in MPL and GPL (units of IgM-aCL and IgG-aCL, respectively). Twenty GPL units/mL for IgG and 20 MPL units/mL for IgM were considered to be positive results.
Patients with platelet counts < 50,000/µL were placed on prednisolone (PD) therapy. PD was administered at a dose of 1 mg/kg/day for 4-6 weeks, and then was tapered. In patients with initial platelet counts < 20,000/µL, intravenous immune globulin (IVIg) was administered at a dose of 400 mg/kg/day for 5 days in combination with PD. Splenectomy was performed in patients who were refractory to PD. Response was defined as 1) a platelet count ≥ 100,000/µL at the last clinic visit, when the initial platelet count was > 50,000/µL; or 2) a platelet count increase of ≥ 20,000/µL from baseline to a final value > 50,000/µL. Responses lasting < 3 and ≥ 3 months after the initiation of treatment were defined as transient and sustained responses, respectively.
Eligible patients were monitored every 1-2 weeks for the first 3 months and then every 1-3 months for the remaining period, to conduct laboratory investigations and check for the development of arterial or venous thrombotic events. A complete blood count was determined at every visit. Routine chemistry, urine analysis, studies to exclude SLE, and aPL tests were performed 12 months apart.
Characteristics, response to treatment, and frequency of thrombosis were compared between aPL-positive and -negative patients using the chi-square test as appropriate for categorical variables and Student's t test for continuous variables. A p value < 0.05 was considered to indicate significance. All analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Seventy patients were enrolled. The median age was 48 years (range, 18 to 79), and 45 patients (64.3%) were female. Most of the patients (91.4%) had platelet counts < 50,000/µL (Table 1). Of these, aPL (aCL and LA) were detected in 20 patients (28.5%): aCL alone in 15 (75%), aCL and LA in two (10%), and LA alone in three (15%). Of the 15 patients who were positive for aCL only, eight had IgG-aCL only, four had IgM-aCL only, and three had both IgM- and IgG-aCL. LA was detected in a total of five patients and was associated with aCL in two (Table 2). Age, gender, and platelet count did not differ between the aPL-positive and -negative groups (Table 1).
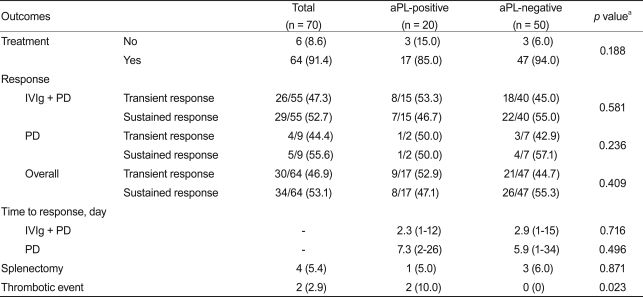
Sixty-four of the 70 patients (17 aPL-positive; 47 aPL-negative) received PD therapy with or without IVIg. Six of the 70 (those with initial platelet counts > 50,000/µL) did not receive any therapy. All patients who received therapy exhibited a transient or sustained response. Both transient and sustained response rates were similar between the aPL-positive and -negative groups (Table 3). The time to response did not differ between the aPL-positive and -negative groups, regardless of treatment modality (Table 3).
Most patients (88.5%; 62 of 70) were followed with aPL tests 12 months apart, 85.0% (17 of 20) in the aPL-positive group and 90.0% (45 of 50) in the aPL-negative group. No patient who was aPL-positive at the time of ITP diagnosis lost aPL positivity, and none of those who were aPL-negative at the time of ITP diagnosis displayed aPL during follow-up (data not shown).
The median follow-up periods in the aPL-positive and -negative groups were 19.6 (interquartile range, 15.5 to 27.5) and 20.7 (18.7 to 28.1) months, respectively. The 50 patients who did not have aPL at diagnosis did not display thrombotic events during a median follow-up of 20 months (range, 2 to 68). In contrast, two of the 20 aPL-positive patients (11%) experienced thrombotic events (Table 3).
A 54-year-old man who had atrial fibrillation developed acute myocardial infarction 2 months after diagnosis of ITP. Platelet counts at diagnosis and the thrombotic event were 39,000/µL and 61,000/µL, respectively. He had IgM-aCL, but not LA or IgG-aCL, at diagnosis of ITP. He had discontinued PD 2 weeks before the thrombotic episode. He underwent successful intracoronary stenting and received aspirin as prophylaxis. Another patient, a 56-year-old woman, had LA, a high level of IgM-aCL (29.7 MPL units/mL), and obesity. She developed deep vein thrombosis (DVT) 5 months after the diagnosis of ITP. Her platelet counts at diagnosis and the thrombotic event were 31,000/mL and 12,000/µL, respectively. She was taking PD at the time of development of DVT. No patient received IVIg, danazol, or underwent splenectomy during the 2 weeks preceding the thrombotic events (Table 4). Although the two patients had risk factors other than aPL for thrombosis, thrombotic risk factors did not differ as a function of aPL status (Table 5).
A variable number of patients with ITP have aPL at the time of diagnosis and during follow-up after treatment. In the present study, more than one-fourth of patients with ITP (28.5%) had aPL at the time of diagnosis. This was similar to or lower than previously reported rates [5-8,10-13]. The reason underlying this discrepancy is not clear. Additionally, controversy still exists regarding the clinical significance of aPL in these patients. Nevertheless, bleeding manifestations, platelet counts, and response to corticosteroid treatment have been repeatedly demonstrated to not differ as a function of aPL status at the time of diagnosis. The present study confirmed that the presence or absence of aPL was not associated with a specific clinical presentation and did not affect treatment responses in patients with ITP.
Thrombocytopenia has been reported to have a prevalence of 20 to 46% in patients with primary APS [5-7]. Since the first definitive clinical and pathological description of APS [18], the association between aPL and the development of thrombosis has been extensively investigated over decades in patients with APS. However, previous studies of aPL in ITP have been limited, compared with those in APS, and only a few clinical studies have examined the relationship between aPL positivity and risk for thrombosis. A prospective study on 149 patients with ITP could identify no relationship between thrombosis and aPL positivity [8]. In contrast, a similarly designed study involving 82 consecutive patients with ITP reported that aPL-positive patients had a greater incidence of thrombosis over a 5-year period (61 vs. 2.3%) [6]. In another study on 216 patients with ITP, 14 of 55 (25%) aPL-positive patients developed thrombosis over a 2.5-year period [13]. In that study, investigators emphasized that thrombosis was associated with LA, a high IgG-aCL level, and younger age (i.e., < 45 years) at the time of thrombosis. We found that two of 20 patients (10%) who were initially positive for aPL experienced thrombosis during follow-up. One patient had venous thromboembolism, and the other had arterial thrombosis. No correlation was found between thrombotic events and types of aPL, platelet count, steroid therapy, or age. Patients who were initially negative for aPL never experienced thrombosis. These results provide evidence of an increased risk for thromboembolic events in adult aPL-positive ITP patients compared with aPL-negative ITP patients. Contrary to some early reports, individuals with elevated aPL showed a much lower rate of thrombosis in the present study. This may be attributable to the relatively short follow-up duration and comparatively small size of the patient population. It is difficult to discuss the relationship between steroid use and thrombotic events for the same reasons. Whether prophylactic antithrombotic treatment is beneficial in aPL-positive ITP patients remains uncertain, and prospective studies are required.
In summary, the presence of aPL is a common finding in Korean populations with ITP who share similar clinical profiles regardless of aPL status. Although the risk for thrombosis was not high, the development of thrombosis in aPL-positive, but not aPL-negative, patients suggests that measurement of aPL should be considered in every newly diagnosed ITP patient. Additional studies are needed to determine whether prophylactic anticoagulation treatment is beneficial for aPL-positive patients.
Acknowledgments
This study was financially supported in 2006 by the research fund of Chungnam National University.
References
1. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168–186PMID : 19846889.


2. Font J, Lopez-Soto A, Cervera R, et al. The 'primary' antiphospholipid syndrome: antiphospholipid antibody pattern and clinical features of a series of 23 patients. Autoimmunity 1991;9:69–75PMID : 1669849.


3. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306PMID : 16420554.


4. Udayakumar N, Ezhilan N, Rajendiran C. Primary antiphospholipid antibody syndrome: how certain can you be of the diagnosis? J Clin Rheumatol 2007;13:53. PMID : 17278956.


5. Ballerini G, Gemmati D, Moratelli S, Morelli P, Serino ML. Anticardiolipin antibody-related thrombocytopenia: persistent remission after splenectomy. Haematologica 1995;80:248–251PMID : 7672720.

6. Diz-Kucukkaya R, Hacihanefioglu A, Yenerel M, et al. Antiphospholipid antibodies and antiphospholipid syndrome in patients presenting with immune thrombocytopenic purpura: a prospective cohort study. Blood 2001;98:1760–1764PMID : 11535509.


7. Bidot CJ, Jy W, Horstman LL, Ahn ER, Yaniz M, Ahn YS. Antiphospholipid antibodies (APLA) in immune thrombocytopenic purpura (ITP) and antiphospholipid syndrome (APS). Am J Hematol 2006;81:391–396PMID : 16680753.


8. Arfors L, Winiarski J, Lefvert AK. Prevalence of antibodies to cardiolipin in chronic ITP and reactivity with platelet membranes. Eur J Haematol 1996;56:230–234PMID : 8641391.


9. Font J, Cervera R, Lopez-Soto A, et al. Anticardiolipin antibodies in patients with autoimmune diseases: isotype distribution and clinical associations. Clin Rheumatol 1989;8:475–483PMID : 2612116.


10. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009;114:937–951PMID : 19357394.


11. Stasi R, Stipa E, Masi M, et al. Prevalence and clinical significance of elevated antiphospholipid antibodies in patients with idiopathic thrombocytopenic purpura. Blood 1994;84:4203–4208PMID : 7994034.


12. Kaburaki J, Kuwana M, Ikeda Y. Anti-cardiolipin-beta2-GPI complex antibodies in idiopathic thrombocytopenic purpura. Intern Med 1998;37:796. PMID : 9804093.

13. Pierrot-Deseilligny Despujol C, Michel M, Khellaf m, et al. Antiphospholipid antibodies in adults with immune thrombocytopenic purpura. Br J Haematol 2008;142:638–643PMID : 18510681.


14. George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood 1996;88:3–40PMID : 8704187.


16. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. PMID : 9324032.

17. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009;7:1737–1740PMID : 19624461.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



