INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease that is characterized by chronic inflammatory and destructive polyarthritis. The low oxygen tension of the RA synovial fluid is a potent stimulus for angiogenesis. Angiogenesis is one of the key mechanisms for maintaining and perpetuating chronic inflammation in RA, and the expression of angiogenic mediators in the RA synovium has been described. Interleukin (IL)-8, which was originally identified as a neutrophil chemoattractant, is now known to be a key factor in the angiogenesis of human cancers [1-3]. Vascular endothelial growth factor (VEGF) is a major pro-angiogenic growth factor that is produced by activated synovial fibroblasts in rheumatoid joints [4,5].
Toll-like receptors (TLRs) comprise a family of germline-encoded type I transmembrane proteins that enable recognition of pathogen-associated molecular patterns (PAMPs) by the innate immune system [6]. In mammals, more than ten TLR family members are known to recognize specific components that are conserved among microorganisms. TLRs function as pathogen-recognition receptors (PRRs), which primarily sense the PAMPs found in a wide variety of pathogens, including bacteria, fungi, protozoa, and viruses [7]. Although only a few studies have investigated the roles of TLRs in RA pathogenesis, they are likely to have complex roles in this disease. TLR2, TLR3, TLR4, and TLR7 are highly expressed in the RA synovium [8-10], TLR3 is highly expressed in fibroblast-like synoviocytes (FLS), and TLR2 and TLR4 are expressed by the CD14+ macrophages and peripheral blood cells from patients with RA. Double-stranded RNA (dsRNA), which is produced by many viruses during replication, is a common viral PAMP that is recognized by TLR3, whereas single-stranded RNA activates TLR8. In addition to its recognition of dsRNA, TLR3 has the unusual feature that it is preferentially expressed by immature dendritic cells (DC) [11]. Polyinosinic : polycytidylic acid [poly (I:C)], which is a TLR3 ligand, is known to interact with TLR3 expressed on DC. Several studies have been performed to clarify the role of TLR3 signaling in RA pathogenesis [8]. Injection of dsRNA into mice caused a self-limiting arthritis, suggesting that TLR3 signaling contributes to the pathogenesis of arthritis [12]. It has been shown that retinal pigmented epithelial cells produce more IL-6, IL-8, monocyte chemotactic protein (MCP-1), and intercellular adhesion molecule-1 (ICAM-1) after TLR3 ligation by poly (I:C) [13]. Therefore, it is conceivable that TLR3 signaling functions in angiogenesis in the rheumatoid synovium, although the details of this role remain to be elucidated.
To determine the role of TLR3 signaling in angiogenesis in the rheumatoid synovium, we investigated whether the expression levels of two representative pro-angiogenic molecules, VEGF and IL-8, were up-regulated by TLR3 ligation in human RA FLS. We also identified the intracellular signaling pathway that mediates TLR3 ligation-induced upregulation of pro-angiogenic molecules in RA FLS.
METHODS
Isolation of FLS
FLS were isolated by enzymatic digestion of synovial tissues obtained from patients with RA or osteoarthritis (OA) who were undergoing total knee replacement surgery, as described previously [14]. The Medical Ethics Committee of the Catholic University of Korea approved the experimental protocol.
Reagents
The TLR3 ligand poly (I:C), prolinedithiocarbamate (PDTC), and parthenolide were purchased from Sigma Chemical Co. (St. Louis, MO, USA). PDTC and parthenolide are nuclear factor-kappaB (NF-╬║B) inhibitors.
Immunohistochemical staining of RA and OA synovial tissues
Immunohistochemical staining for TLR3, VEGF, and IL-8 was performed on sections of the RA and OA synovium. Briefly, the samples of synovium were fixed in 4% paraformaldehyde solution overnight at 4Ōäā, dehydrated with alcohol, washed, embedded in paraffin, and sectioned into 7-┬Ąm-thick slices. Endogenous peroxidase activity was blocked by treatment with 3% H2O2.
After overnight incubation at 4Ōäā with the primary anti-IL-8 antibody, anti-VEGF antibody (Vector Laboratories, Burlingame, VT, USA) or anti-human TLR3 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), the samples were incubated with the biotinylated secondary linking antibodies for 2 hours and streptavidin-peroxidase complex for 1 hour, followed by incubation with 3,3'-diaminobenzidine (DAKO, Glostrup, Denmark) for 5 minutes.
Measurements of IL-8 and VEGF transcripts by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR)
FLS were incubated with a range of poly (I:C) concentrations (0.1 ┬Ąg/mL to 50 ┬Ąg/mL). After 12 hours of incubation, mRNA was extracted using RNAzol B (Biotex Laboratories, Houston, TX, USA) according to the manufacturer' instructions. Reverse transcription of 2 ┬Ąg total mRNA was carried out at 42Ōäā using the SuperscriptŌäó reverse transcription system (Takara, Shiga, Japan). PCR amplification of a cDNA aliquot was performed by adding 2.5 mM dNTPs, 2.5 U Taq DNA polymerase (Takara), and 0.25 ┬ĄM each of the sense and antisense primers. The following primers (sense and antisense, respectively) were used: for VEGF, 5'-TCTTGGGTGCATTGGAGCCTC-3' and 5'-AGCTCATCTCTCCTATGTGC-3'; and for IL-8, 5'-CCTGATTTCTGCAGCTCTGT-3' and 5'-AACTTCTCCACAACCCTCTG-3'. The reactions were processed in a DNA thermal cycler (Perkin-Elmer Cetus, Wellesley, MA, USA) using the following conditions: for IL-8, 35 cycles of 94Ōäā for 30 seconds, 58Ōäā for 30 seconds, and 72Ōäā for 30 seconds; for VEGF, 33 cycles of 94Ōäā for 30 seconds, 55Ōäā for 30 seconds, and 72Ōäā for 30 seconds; and for GAPDH, 25 cycles of 94Ōäā for 30 seconds, 56Ōäā for 30 seconds, and 72Ōäā for 30 seconds.
The levels of VEGF mRNA and IL-8 mRNAs were determined by real-time PCR using SYBR green I. For quantitative analysis of the VEGF and IL-8 transcripts, the LightCyclerŌäó (Roche Diagnostics, Mannheim, Germany) was used. The relative expression levels of the samples were calculated from the levels of VEGF and IL-8 transcripts normalized to the value for the endogenously expressed house-keeping gene GAPDH. Melting curve analysis was performed immediately after amplification under the following conditions: 0 second (hold time) at 95Ōäā; 15 seconds at 65Ōäā, and 0 second (hold time) at 95Ōäā. The rate of temperature change was 20Ōäā/sec, except in the final step, in which the rate was 0.1Ōäā/sec. The crossing-point (Cp) was defined as the maximum of the second derivative from the fluorescence curve.
RESULTS
Effects of TLR3 ligation on VEGF mRNA and IL-8 mRNA expression in RA FLS
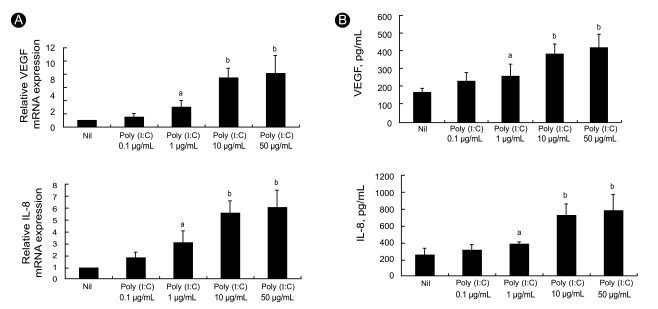
Since angiogenesis is an important feature of synovitis and promotes leukocyte influx into joints, we examined two key pro-angiogenic molecules. We measured the levels of VEGF and IL-8 mRNA expression in RA FLS after 12 hours of treatment in vitro with the TLR3 ligand poly (I:C), which is a synthetic dsRNA analog. Semiquantitative RT-PCR analyses showed that 12 hours of stimulation with poly (I:C) induced high levels of VEGF and IL-8 transcripts in the RA FLS (Fig. 1A). The levels of both transcripts were increased in a dose-dependent manner as the poly (I:C) concentration was increased from 0.1 ┬Ąg/mL to 50 ┬Ąg/mL.
Increased production of VEGF and IL-8 by RA FLS after TLR3 ligation
To ascertain the production of angiogenic factors by RA FLS following TLR3 stimulation for 48 hours, we measured the levels of VEGF and IL-8 in the culture supernatants using sandwich ELISAs. The production levels of VEGF and IL-8 were strongly up-regulated in a dose-dependent manner by stimulation with poly (I:C) (Fig. 1B).
Expression of TLR3, VEGF, and IL-8 in the RA and OA synovia
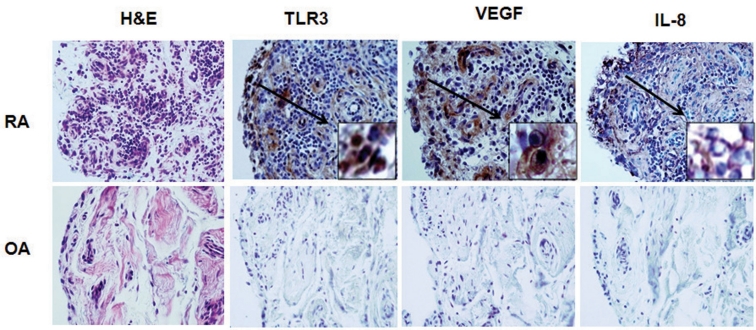
Immunohistochemical staining revealed constitutive expression of TLR3, VEGF, and IL-8 in the RA synovium, and these levels were higher than those in the OA synovium (Fig. 2). TLR3- and VEGF-expressing cells were mainly located in the synovial lining layer. IL-8 expression was observed both in the sublining perivascular area and scattered among the synovial lining cells.
TLR3 ligation mediates VEGF and IL-8 production through NF-╬║B signaling in RA FLS
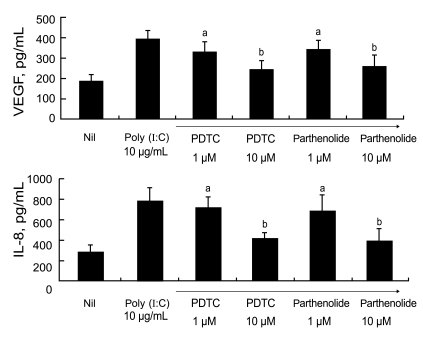
It is well known that the transcription factor NF-╬║B participates in TLR3 signaling, and both PDTC and parthenolide are commonly used as inhibitors of NF-╬║B activity. To elucidate the signal involved in TLR3 ligation-mediated production of VEGF and IL-8, we added various concentrations of PDTC or parthenolide to the RA FLS cultures. After 48 hours, we measured the levels of VEGF and IL-8 in the culture supernatants by ELISA.
Fig. 3 shows that the augmented production of VEGF and IL-8 after stimulation with 10 ┬Ąg/mL poly (I:C) was reduced after treatment with 1 ┬ĄM PDTC or 1 ┬ĄM parthenolide. The reductions in the levels of pro-angiogenic molecules were inhibitor dose-dependent. In summary, TLR3 ligation in RA FLS results in increases in VEGF and IL-8 production, which are mediated by the NF-╬║B signaling pathway.
DISCUSSION
Histologically, synovial tissues from patients with RA are rich in new vascular formations, which promote formation of the pannus that invades adjacent cartilage and bony structures. Although neo-angiogenesis is observed in the earliest phase of the disease, the presence or density of new vessels is significantly increased in patients with disease of longer duration, higher activity, and greater severity [15]. As newly formed blood vessels supply oxygen and nutrients to proliferating synovial cells, angiogenesis plays a key role in maintaining the chronic inflammatory response in RA [16]. These processes are augmented by pro-angiogenic molecules, the most potent of which is VEGF [4,16]. Many chemokines have been detected in the rheumatoid joint. IL-8 (IL-8/CXCL8), which is a C-X-C chemokine that was originally characterized as a potent chemoattractant for neutrophils, contributes to the influx of polymorphonuclear cells into the joint. In addition, IL-8 shares with VEGF proangiogenic properties for vascular endothelial cells [17]. IL-8 modulates multiple biologic functions via the CXCR1 and CXCR2 expressed on endothelial cells. Several reports have suggested that IL-8 directly modulates endothelial proliferation and migration, and regulates angiogenesis in vitro as well as in vivo [18-20].
TLRs are involved in both innate and adaptive immune responses, and play complex roles in the pathogenesis of human RA and experimental arthritis [21,22]. Unlike other members of the TLR family, such as TLRs 2, 4, 7, and 9, which have LPS or unmethylated CpG motifs as ligands, TLR3 reacts with the dsRNA from infecting viruses. This dsRNA, which represents a prototypic viral conformation of nucleic acid, appears to be highly arthritogenic in mice. In fact, viral infection is known to exacerbate RA joint inflammation during the course of disease [23]. Bokarewa et al. [24] have shown that patients with RA who have an erosive disease course have significantly higher levels of dsRNA in their synovial fluids than patients with non-erosive disease.
Brentano et al. [8] have reported that mRNA-containing necrotic cell debris, which is released from cells in the RA synovial fluid, acts as an endogenous TLR3 ligand in RA FLS. As a consequence of TLR3 engagement, the production levels of pro-inflammatory cytokines and chemokines, including interferon (IFN)-╬▓, CXCL10, CCL5, and IL-6, are increased. In this respect, TLR3 expression in inflammatory joints has been correlated with the presence of IL-1╬▓, IL-18, and IFN-╬│ [25]. These data suggest the importance of TLR3 ligation in chronically inflamed RA joint tissues. In addition, a recent study [26] has shown that the TLR3 ligand poly (I:C) directly induces the synthesis of IL-17A and IL-21 in human T-helper cells. Both of these cytokines have been implicated in the pathogenesis of RA.
Regarding TLR3 and angiogenesis, papers have been published that suggest potent effects of TLR3 on angiogenesis in prostate cancer [27] and choroidal neovascularization [28]. The aim of the present study was to assess the effect of TLR3 engagement on angiogenesis, which is one of the fundamental processes in the evolution of the inflammatory response in RA synovial joints.
Our previous studies [29] revealed that TLR3 enhances osteoclastogenesis through upregulation of receptor activator of NF-╬║B ligand (RANKL) expression in RA FLS. In the present study, we investigated the effects of TLR3 signaling in RA FLS on two key pro-angiogenic molecules, VEGF and IL-8. Our data demonstrate that TLR3 stimulation by poly (I:C) augments the expression of VEGF and IL-8 at both the mRNA and protein levels in RA FLS, and suggest that TLR3 induces these activities via the NF-╬║B signaling pathway.
These findings imply that viral particles or the necrotic cell debris in RA synovial fluid increase the expression of these pro-angiogenic molecules and participate in new vessel formation in patients with RA. Combined with other previous studies, our findings support the notion that viral infection either initiates or facilitates the development of autoimmunity, based on the observation that viral RNA is a ligand for TLR3.
Unfortunately, we could not demonstrate an in vivo effect on angiogenesis of TLR3 ligation on RA FLS. A limitation of the present study is that we show increased expression of two pro-angiogenic factors, rather than a change in angiogenesis itself.
In summary, our results suggest that the expression levels of VEGF and IL-8 transcripts and proteins are up-regulated in response to poly (I:C), a synthetic TLR3 ligand, through the NF-╬║B signaling pathway. Angiogenesis is an early and critical event in the pathogenesis in RA, and recent evidence suggests that aggressive treatment during the very early stages of RA can be beneficial, implying the existence of a critical therapeutic window of opportunity [30]. Effective blockade of specific TLR signaling is a potential therapeutic option to prevent progression to joint destruction.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





