 |
 |
| Korean J Intern Med > Volume 25(2); 2010 > Article |
|
Abstract
A 59-year-old man was admitted with numbness, pain, and a tingling sensation in both lower legs. He was initially diagnosed with diabetic peripheral neuropathy based on a symptom questionnaire and a quantitative sensory test. Despite symptomatic treatment of diabetic neuropathy, he complained of worsening sensory symptoms and additional motor weakness in both lower extremities. As the motor weakness of both extremities became more aggravated over time, brain and spine imaging tests and a nerve conduction test were performed. The nerve conduction study revealed motor and sensory axonal neuropathy. In his cerebrospinal analysis, albumino-cytologic dissociation, which is compatible to the Gillian-Barre syndrome, was found. Cerebrospinal fluid analysis showed albumino-cytologic dissociation. He was treated with intravenous immunoglobulin and his neurologic deficits were gradually improved.
Neuropathy in diabetic patients is heterogenous, so can present with many sensory and motor symptom [1]. Diverse neuropathic symptoms are important in the early recognition and diagnosis of diabetic neuropathy (DN). If the neurological symptoms are atypical and are not resolved, especially when motor neurons were involved, other causes should be considered because DN is an exclusive diagnosis and nondiabetic neuropathy may be present in diabetic patients [2]. Among these, Guillain-Barre syndrome (GBS) is a disease that should be considered when acute flaccid weakness occurs [3].
We report here a case of diabetic neuropathy combined with GBS, which was diagnosed with some delay due to the underlying diabetes.
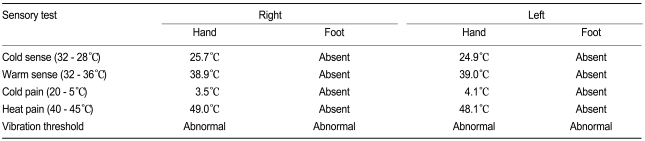
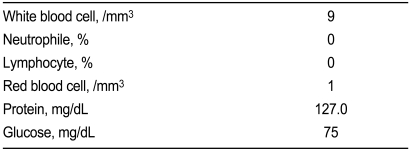
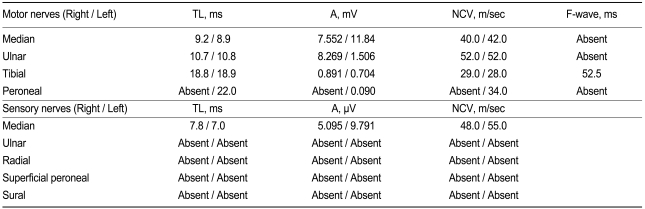
A 59-year-old man complained of pain and numbness in both lower legs of several months duration. His medical history was diabetes of 15 years and he recently had caught a common cold. He had no significant neurological history and received insulin treatment for glucose control. Laboratory findings showed: proteinuria (+), glucosuria (++), hemoglobin 10.1 g/dL, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) 24/30 IU/L, creatinine kinase (CK) 64 U/L, glucose 288 mg/dL, Na+/K+/Cl- 130/4.1/98 mmol/L, blood urae nitrogen (BUN)/creatinine (Cr) 28/1.2 mg/dL, HbA1c 8.8%, C-peptide 1.2 ng/mL, and total cholesterol 248 mg/dL. On the neurological examination, there were no abnormal findings for the motor response. After admission, the patient was treated with insulin for glucose control and alpha-lipoic acid (ALA), gabapentin, and amytryptilin for diabetic peripheral neuropathy based on symptoms (total symptom score, 9.65), 10 g monofilament test, and quantitative sensory test (Table 1). After two days, however, the sensory symptoms in both legs were more aggravated and there was additional motor weakness in the lower legs. These symptoms were not present before admission. On day 4, he could not walk and had difficulty sensing movement of upper extremities, although he had not had any discomfort in ambulation at the visiting hospital. Cervical and brain MRI were performed but resulted in near normal findings except for mild herniation of an intervertebral disc. On day 5, aggravated weakness of the lower legs had spread to the upper extremities, so we consulted with the neurology department. Deep tendon reflex was absent and so cerebrospinal fluid (CSF) examination and a neurophysiological study were performed. The CSF showed albumino-cytologic dissociation (Table 2) and the electrodiagnostic finding was acute motor and sensory axonal neuropathy compatible with GBS (Table 3). Immunoglobulin was the administered intravenously and the neurological deficit of motor weakness improved gradually over the next week, allowing the patient to be discharged. The sensory discomforts however, such as pain, numbness, and tingling sense, which were thought to be caused by DN, were little relieved with continuous medication of ALA and gabapentin.
Many diabetic patients suffer from neuropathic symptoms involving sensory and motor nerves [1,4]. However, motor neuron involvement including paralysis or weakness of extremities is not common in DN. Generally confirmation of DN can be established by neuropathic symptoms, sensory and autonomic function test, and quantitative electrophysiology. Nevertheless, other causes should be excluded [2]. Cerebrovascular accident, peripheral vascular insufficiency, spinal disease, periodic paralysis, myasthenia, transverse myelitis, toxic neuropathy, diabetic peripheral neuropathy, and GBS are all possible causes [5,6].
Since the prognosis of the neuropathic patient sometimes is dependent on early recognition and prompt treatment of the cause, an exact diagnosis is needed in the early period of the disease. To avoid misdiagnosis when the patient suffers from neuropathic symptoms combined with acute weakness or paralysis of limbs, the physician must be aware of the possibility of GBS, even if the patient is being treated for another disease. Although there are no reports show of a high risk of GBS in diabetic patients, a few cases of GBS associated with diabetic ketoacidosis have been described [7]. GBS underlying DN is uncommon but could possibly be a serious cause of paralysis or weakness of limbs, although this condition can be overlooked on presentation. Prompt and sufficient evaluation and management should be done in the early stages when patients are suffering from neuropathic symptoms which are not explained by previously diagnosed underlying disease.
GBS is a treatable disease which is associated with morbidity and mortality if untreated [8]. GBS is the commonest peripheral neuropathy affecting children but it can occur at any age and condition. It is characterized by rapidly progressing symmetric limb weakness, loss of tendon reflexes and sensory signs, and autonomic dysfunction [9]. Diverse symptoms in GBS can occur according to the subtypes. Acute peripheral neuropathy in GBS includes acute inflammatory demyelinating polyradiculoneuropathy, acute motor axonal neuropathy, and acute motor and sensory axonal neuropathy [10]. The onset of neurological symptoms, such as flaccid weakness of lower limbs, is usually sudden, but is possible anytime between 1 to 28 days after the prodromal event. Weakness of the limbs is usually present bilaterally with symmetrical involvement spreading upwards to the arms, but asymmetrical presentation and only distal involvement of the legs are also possible [3]. Although the exact pathogenic mechanisms are uncertain, there is considerable evidence that an acute demyelinating process occurs by an immunemediated reaction with neural antigen after various viral illnesses including herpes, mumps, mycoplasma, cytomegalovirus and Campylobacter or after immunization [3,11].
For the diagnosis of GBS, CSF findings of increased protein and normal cell count is helpful. In addition, neurophysiological examinations including nerve conduction studies are essential for the confirmation of GBS. Marked slowing of motor conduction velocity, prolonged distal latency, and conduction block can be shown in nerve conduction studies. Early symptomatic and supportive treatments including careful monitoring of vital signs are the mainstay of the management. Until now plasma exchange or intravenous immunoglobulin have been recommended for effective treatment [12]. Several factors such as occurrence in adulthood, rapid development of paralysis, delay in the diagnosis, marked distal involvement, a low amplitude or fibrillation pattern in the electromyographic potential have been suggested as predictors of poor prognosis [3].
In our case, due to diagnostic delay, rapid progression and electromyografic findings, a poor prognosis was predicted. However, near complete recovery was achieved. We report a case of DN combined with overlooked GBS that was detected with delay to encourage prudence in caring for neuropathic diabetic patients.
References
1. Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care 2004;27:1458–1486PMID : 15161806.


2. Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med 1998;15:508–514PMID : 9632127.


3. Stewart JD, McKelvey R, Durcan L, Carpenter S, Karpati G. Chronic inflammatory demyelinating polyneuropathy (CIDP) in diabetics. J Neurol Sci 1996;142:59–64PMID : 8902721.


4. Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962PMID : 15793206.


5. Woolf CJ. American College of Physicians. American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 2004;140:441–451PMID : 15023710.


6. Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain 2002;18:343–349PMID : 12441827.


7. Rouanet-Larriviere M, Vital C, Arne P, Faverel-Garrigues JC, Gin H, Vital A. Guillain-Barré syndrome occurring in two women after ketoacidosic comatose state disclosing an insulin-dependent diabetes mellitus. J Peripher Nerv Syst 2000;5:27–31PMID : 10780681.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



