 |
 |
| Korean J Intern Med > Volume 25(1); 2010 > Article |
|
Abstract
Background/Aims
To characterize ultrasonographic findings in papillary thyroid carcinoma (PTC) combined with Graves' disease.
Methods
Medical records and ultrasonographic findings of 1,013 patients with Graves' disease and 3,380 patients without Graves' disease were analyzed retrospectively. A diagnosis of PTC was based on a pathologic examination.
Results
The frequency of hypoechogenicity was lower in patients with PTC and Graves' disease than in patients with PTC alone (p < 0.05). The frequency of perinodular blood flow in patients with PTC and Graves' disease was significantly higher than in those with PTC alone (p < 0.05). PTC combined with Graves' disease was characterized by more ill-defined borders and less frequency of overall calcification, punctate calcification, and heterogeneous echogenicity, although the difference was not statistically significant.
Because thyroid ultrasonography is the most sensitive method to detect thyroid nodules [1], cytological examination guided by ultrasonography has better diagnostic accuracy than palpation [2]. Application of these methods reveals many thyroid cancers that are not detected by routine clinical examinations. Many studies have reported ultrasonographic features suggesting thyroid cancer in general [3-7].
The use of ultrasonography to evaluate the thyroid gland has resulted in the detection of a large number of non-palpable thyroid nodules in patients with Graves' disease. Papillary thyroid carcinoma (PTC) often occurs in Graves' patients [8-10]. However, it is often difficult to differentiate malignant and benign nodules in this setting, because the appearance of Graves' disease varies on ultrasonography [11].
In addition to frank neoplasm, Graves' disease itself may cause thyroid nodularity, due to coexisting colloid goiter, autoimmune lymphocytic disease, degenerative-involutional changes, and hyperplastic adenomatous tissue [12]. Thus, the application of ultrasonography for the detection of thyroid cancer in Graves' patients may be problematic in clinical practice.
The aim of this study was to characterize ultrasonographic findings of PTC in patients with Graves' disease.
We retrospectively reviewed the records of 1,013 patients with Graves' disease (749 women, 264 men) and 3,380 patients without Graves' disease (2,704 women, 676 men) who underwent ultrasonographic examinations between January 2003 and December 2007. A diagnosis of Graves' disease was made when there was a diffuse thyroid goiter with positive serum thyrotropin-binding inhibitory immunoglobulin (TBII) or anti-thyroid autoantibodies in patients with biochemical and clinical thyrotoxicosis. None of the patients had a history of previous neck irradiation or radioactive iodine therapy. Patients with Hashimoto's thyroiditis were excluded. Graves' patients who were euthyroid or who were treated with anti-thyroid drugs for more than 4 weeks were excluded. The diagnosis of PTC was based on pathologic examination of surgically resected neoplasms. The study plan was reviewed and approved by our institutional ethical committee.
Ultrasonography was performed using a 10-MHz linear probe (LOGIQ 7, GE Medical Systems, Milwaukee, WI, USA). The following ultrasonographic parameters were assessed: nodule composition (completely solid, predominantly solid, mixed solid and cystic, predominantly cystic, or completely cystic); echogenicity (hypoechoic, isoechoic, or hyperechoic); homogeneity (homogeneous or heterogeneous); calcifications (punctuate, rim, nodular, or linear); nodule margin (welldefined or ill-defined); nodule shape (regular or irregular); echographic dimension (anteroposterior and transverse diameter); peripheral hypoechoic rim (thin or thick), and nodular vascularity (perinodular or intranodular).
We assessed nodule shape according to the ratio of the anteroposterior dimension to the transverse dimension (A/T ratio) as Ōēź 1 or < 1. Nodule composition was classified based on the approximate portion of the nodule that was cystic as follows: completely solid, predominantly solid (1 - 24% cystic), mixed solid and cystic (25 - 74% cystic), predominantly cystic (75 - 99% cystic), or completely cystic. Echogenicity was categorized with respect to the background of the surrounding parts of the thyroid gland. Calcification was categorized as punctuate calcification and macrocalcification. Punctuate calcifications (microcalcifications) were defined as tiny, punctuate hyperechoic foci that were usually less than 2 mm in size, regardless of any posterior acoustic shadowing. Rimmed, nodular, or linear calcifications were classified as macrocalcifications. A hypoechoic rim was defined as a low echoic rim surrounding the nodule. A rim greater than 2 mm was classified as thick. Vascularity, as determined by power Doppler ultrasonographic imaging, was defined as absent or not apparent, perinodular alone, or intranodular. If both perinodular and intranodular vascularity were identified, the nodule was characterized as having intranodular vascularity.
Serum levels of thyroid hormone (T3, free T4) and thyroid stimulating hormone (TSH) were measured using commercial radioimmunosorbent assay and immunoradiometric assay kits (CIS Bio International, Shering, France), respectively. TBII activity was measured with a radioreceptor assay kit (RSR Ltd., Cardiff, UK), and anti-Thyroglobulin (Tg) and anti-Thyroid peroxidase (TPO) antibodies with enhanced chemo-luminescence (ECL, Roche, Mannheim, Germany).
All data are expressed as means ┬▒ SD. Statistical analyses were performed using the SPSS software (SPSS Inc., Chicago, IL, USA). The significance of differences in categorical variables between groups was tested with the chi-squared test or a linear-by-linear association. Continuous variables were analyzed using the independent t-test. A p value < 0.05 was deemed to indicate statistical significance.
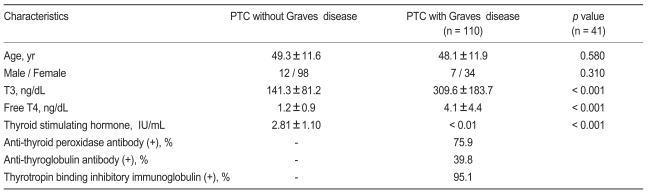
Of 3,380 patients without Graves' disease, 110 were diagnosed with PTC, and of 1,013 Graves' patients, 41 were diagnosed with PTC. Clinical and hormonal characteristics of PTC patients with and without Graves' disease are shown in Table 1. PTC patients with Graves' disease had higher serum T3 and free T4 levels, and lower serum TSH value than PTC patients without Graves' disease (p < 0.001). There was no significant difference in age or gender between the two groups, although females were more prevalent in both groups.
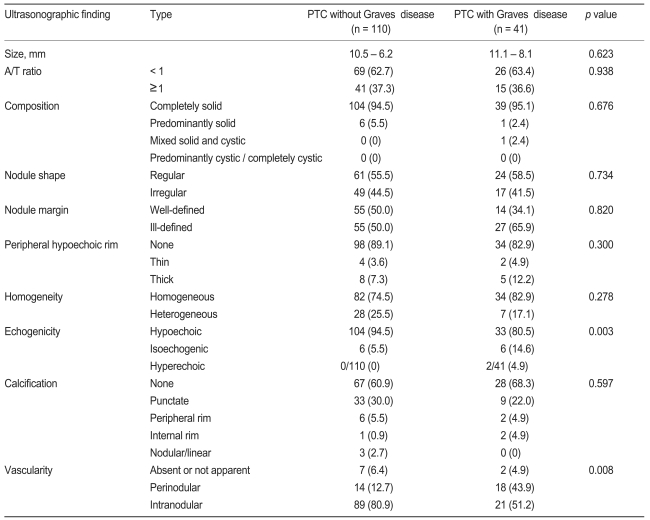
We compared the ultrasonographic findings in the two groups (Table 2). In both groups, many patients demonstrated typical PTC findings, such as completely solid composition, ill-defined border, peripheral hypoechoic rim, hypoechoic echogenicity, and nodular blood flow. Hypoechoic echogenicity was seen in about 81% of PTC patients with Graves' disease and in about 95% of PTC patients without Graves' disease. The frequency of isoechoic or hyperechoic echogenicity was higher in PTC patients with Graves' disease than in PTC patients without Graves' disease (p < 0.05, Figs. 1A and 2A). The frequencies of intranodular and perinodular blood flow in patients with PTC and Graves' disease were 51% and 44%, respectively, and the frequencies of intranodular and perinodular blood flow in patients with PTC alone was 81% and 13%, respectively. The frequency of no nodular blood flow was 5% in PTC patients with Graves' disease, and 6% in PTC patients without Graves' disease (p < 0.05, Figs. 1B and 2B). Most of the PTCs in both groups were composed of completely or predominantly solid elements. There was no difference in calcification pattern between the two groups. Compared with ultrasonographic features suggesting malignancy formation, the frequency of hypoechogenicity in PTC patients with Graves' disease was lower than that seen in PTC patients without Graves' disease (Table 2). The frequency of intranodular flow in PTC patients with Graves' disease was lower than in PTC patients without Graves' disease (Table 2). The frequency of perinodular flow in PTC patients with Graves' disease was significantly higher than in PTC patients without Graves' disease (Table 2). PTC in the presence of Graves' disease was found to have more illdefined borders and less overall calcification, punctate calcification, and heterogeneous echogenicity, but not to a significant degree. There was no significant difference regarding cystic change, peripheral hypoechoic rim, macrocalcifications, or A/T Ōēź 1 (Table 2).
Many studies have examined ultrasonographic features which may be helpful for differential diagnosis of benign and malignant nodules. Thyroid echogenicity reflects the gland's follicular structure. Conditions that modify the normal anatomical structure of the gland cause alterations in echo patterns. In Graves' disease, the echo pattern of the thyroid may undergo changes secondary to the reduced colloid content, with increased cellularity, reduction in the cell-colloid interface, increased vascularity, and lymphocytic infiltration [11]. Thus, it may be important to characterize the ultrasono-graphic features of PTC patients with Graves' disease. However, ultrasonographic findings of thyroid cancer in Graves' disease have not yet been fully determined.
Psammoma body microcalcifications are specific to papillary thyroid cancer [13,14]. Hypoechogenicity and an ill-defined margin are known sonographic findings associated with thyroid cancer [15,16]. Irregular margins, which result from microinvasion of the surrounding tissues, may also indicate thyroid malignancy [13]. Both thick peripheral hypoechoic rim patterns and the absence of a rim surrounding a thyroid nodule, which represents pericapsular inflammatory infiltration, have been reported to be indicators of malignancy [17]. Many studies have demonstrated the potential utility of power Doppler imaging in identifying thyroid nodules with a higher probability of malignancy formation [18]. Recently, several studies have reported that an A/T ratio Ōēź 1 is associated with thyroid malignancy [7,19].
In the present study, ultrasonographic findings of PTC patients both with and without Graves' disease were typical. Hypoechogenicity, ill-defined borders, absence of peripheral hypoechoic rim, no cystic changes, and increased intranodular blood flow were frequently seen in both groups. Punctate calcifications were more common than macrocalcifications in both groups, but there was no significant difference between the groups in this regard.
Many studies have noted that the degree of echogenicity can be used to differentiate thyroid nodules and that most malignant nodules are hypoechoic [16]. Ultrasonic echogenicity of the thyroid gland depends on cellularity and vascularization of the tissue. The hypoechogenic image is thought to represent the cellular microfollicular histological milieu, whereas ultrasonographic findings of a macrofollicular nodule may be isoechoic or hyperechoic [20]. Reduced colloid content, lymphocyte infiltration and increased intrathyroidal flow in Graves' disease lead to hypoechogenic patterns [21]. Graves' disease is also associated with inhomogeneous echogenicity in addition to a higher grade of hypoechogenicity [22]. In the present study, the majority of PTCs without underlying Graves' disease (94.5%) were hypoechoic; only 5.5% were isoechoic. In contrast, 80.5% of PTCs with underlying Graves' disease were hypoechoic, 14.6% were isoechoic, and 4.9% were hyperechoic. This finding may suggest that nonhypoechoic echogenicity of a nodule does not necessarily indicate a low probability of PTC in the presence of Graves' disease.
Tumor growth is associated with cellular proliferation. Cellular proliferation is thought to be correlated with increased vascularization that may be widely variable, up to anarchical angiogenesis, which is present in malignant lesions [23]. Frates et al. [24] reported that increased nodular blood flow was associated with thyroid carcinoma. Carbone et al. [18] proposed that the predominant intranodular blood flow observed in malignant nodules could be explained by the large cellular proliferation in the region. Graves' disease exhibits increased cellularity and an increased vascular pattern, which consists of multiple small areas of intrathyroidal flow, seen diffusely throughout the thyroid gland [21]. Thus, the nodular vascular pattern in the context of Graves' disease might be affected. In the present study, increased intranodular blood flow was frequently seen in PTC patients both with and without Graves' disease. Our results also showed that PTCs in the presence of underlying Graves' disease tended to exhibit significantly increased perinodular hypervascularity (43.9%), as compared to PTCs without underlying Graves' disease (12.7%).
In conclusion, on ultrasonography, the frequency of perinodular hypervascularity was significantly higher, and the frequency of hypoechogenicity was significantly lower, in PTC patients with Graves' disease than in PTC patients without Graves' disease. Thus, our results suggest that patients with Graves' disease more frequently have atypical PTC findings on ultrasonography.
References
1. Bruneton JN, Balu-Maestro C, Marcy PY, Melia P, Mourou MY. Very high frequency (13 MHz) ultrasonographic examination of the normal neck: detection of normal lymph nodes and thyroid nodules. J Ultrasound Med 1994;13:87ŌĆō90PMID : 7932966.


2. Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid 1998;8:15ŌĆō21PMID : 9492148.


3. Hegedus L. Clinical practice: the thyroid nodule. N Engl J Med 2004;351:1764ŌĆō1771PMID : 15496625.


4. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 2002;87:1941ŌĆō1946PMID : 11994321.


5. Nam-Goong IS, Kim HY, Gong G, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004;60:21ŌĆō28PMID : 14678283.


6. Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med 2003;22:1083ŌĆō1090PMID : 14606565.


7. Kim EK, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol 2002;178:687ŌĆō691PMID : 11856699.


8. Dobyns BM, Sheline GE, Workman JB, Tompkins EA, McConahey WM, Becker DV. Malignant and benign neoplasms of the thyroid in patients treated for hyperthyroidism: a report of the cooperative thyrotoxicosis therapy follow-up study. J Clin Endocrinol Metab 1974;38:976ŌĆō998PMID : 4134013.


9. Pacini F, Elisei R, Di Coscio GC, et al. Thyroid carcinoma in thyrotoxic patients treated by surgery. J Endocrinol Invest 1988;11:107ŌĆō112PMID : 3361079.


10. Belfiore A, Russo D, Vigneri R, Filetti S. Grave's disease, thyroid nodules and thyroid cancer. Clin Endocrinol (Oxf) 2001;55:711ŌĆō718PMID : 11895209.


11. Marino M, Chiovato L, Pinchera A. In: DeGroot LJ, Jameson JL, eds. Graves' disease. Endocrinology. 2005;5th ed. Philadelphia: W.B. Saunders, 1995ŌĆō2028.

12. Carnell NE, Valente WA. Thyroid nodules in Graves' disease: classification, characterization, and response to treatment. Thyroid 1998;8:647ŌĆō652PMID : 9737358.


13. Koike E, Noguchi S, Yamashita H, et al. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg 2001;136:334ŌĆō337PMID : 11231857.


14. Takashima S, Fukuda H, Nomura N, Kishimoto H, Kim T, Kobayashi T. Thyroid nodules: re-evaluation with ultrasound. J Clin Ultrasound 1995;23:179ŌĆō184PMID : 7730464.


15. Noma S, Nishimura K, Togashi K, et al. Thyroid gland: MR imaging. Radiology 1987;164:495ŌĆō499PMID : 3602392.


16. Solbiati L, Volterrani L, Rizzatto G, et al. The thyroid gland with low uptake lesions: evaluation by ultrasound. Radiology 1985;155:187ŌĆō191PMID : 3883413.


17. Tessler FN, Tublin ME. Thyroid sonography: current applications and future directions. AJR Am J Roentgenol 1999;173:437ŌĆō443PMID : 10430150.


18. Cerbone G, Spiezia S, Colao A, et al. Power Doppler improves the diagnostic accuracy of color Doppler ultrasonography in cold thyroid nodules: follow-up results. Horm Res 1999;52:19ŌĆō24PMID : 10640895.


19. Cappelli C, Castellano M, Pirola I, et al. Thyroid nodule shape suggests malignancy. Eur J Endocrinol 2006;155:27ŌĆō31PMID : 16793946.


20. Brkljacic B, Cuk V, Tomic-Brzac H, Bence-Zigman Z, Delic-Brkljacic D, Drinkovic I. Ultrasonic evaluation of benign and malignant nodules in echographically multinodular thyroids. J Clin Ultrasound 1994;22:71ŌĆō76PMID : 8132799.


21. Ralls PW, Mayekawa DS, Lee KP, et al. Color-flow Doppler sonography in Graves disease: "thyroid inferno". AJR Am J Roentgenol 1988;150:781ŌĆō784PMID : 3279732.


22. Baldini M, Orsatti A, Bonfanti MT, Castagnone D, Cantalamessa L. Relationship between the sonographic appearance of the thyroid and the clinical course and autoimmune activity of Graves' disease. J Clin Ultrasound 2005;33:381ŌĆō385PMID : 16240426.


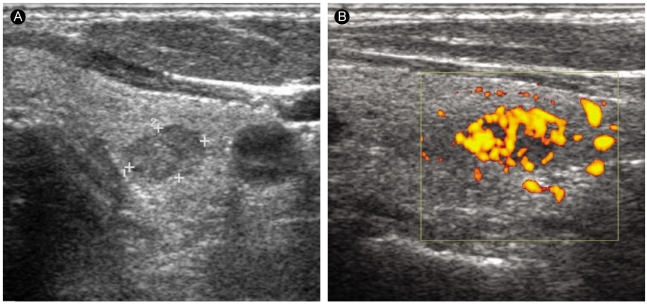
Figure┬Ā1
A 60-yr-old papillary carcinoma patient without Graves' disease. (A) A transverse sonogram of the left thyroid lobe shows a 9-mm hypoechoic nodule. (B) The nodule has perinodular and intranodular hypervascularity.
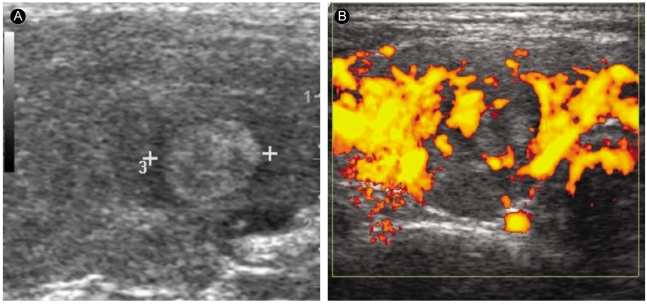
Figure┬Ā2
A 50-yr-old papillary thyroid carcinoma patient with Graves' disease. (A) A longitudinal sonogram of the right thyroid lobe reveals a 10-mm hyperechoic nodule. (B) The nodule has perinodular hypervascularity.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



