 |
 |
| Korean J Intern Med > Volume 22(1); 2007 > Article |
|
Abstract
Inhibitors of tumor necrosis factor-alpha (TNF-╬▒) have been approved for treating rheumatoid arthritis. As one of the biological response modifiers, etanercept has also been used in the treatment of psoriatic arthritis and inflammatory bowel disease. While etanercept is effective, certain infectious complications, such as tuberculosis, fungus, and cytomegalovirus, have been reported. We report the first Korean case of adenoviral pneumonia in a 55-year-old female who developed disseminated adenoviral infection following etanercept treatment, which resolved after anti-TNF-╬▒ discontinuation.
Etanercept is the first anticytokine drug approved to treat rheumatoid arthritis (RA). It is a recombinant-DNA-engineered fusion protein consisting of an extracellular ligand protein domain for tumor necrosis factor-╬▒ (TNF-╬▒) and the constant portion of the human IgG molecule1). Etanercept has demonstrable clinical benefits in several inflammatory arthropathies, including RA and psoriatic arthritis2). Despite trials indicating their safety, the wider use of TNF-╬▒-blocking drugs in patients with RA has led to reports of serious infectious complications. In one study, 5 of 180 patients with rheumatic disease developed the following serious infections while taking etanercept3). acute cholecystitis, septic wrist caused by Staphylococcus aureus, arthroplastic hip infection caused by methicillin-resistant Staphylococcus aureus, psoas abscess caused by Mycobacterium avium-intracellulare, and bacteremia following colonoscopy. Pneumonia caused by parainfluenza virus type 3 has also been reported4). We report the first case of severe adenovirus pneumonia with myocarditis that developed in a patient during etanercept therapy.
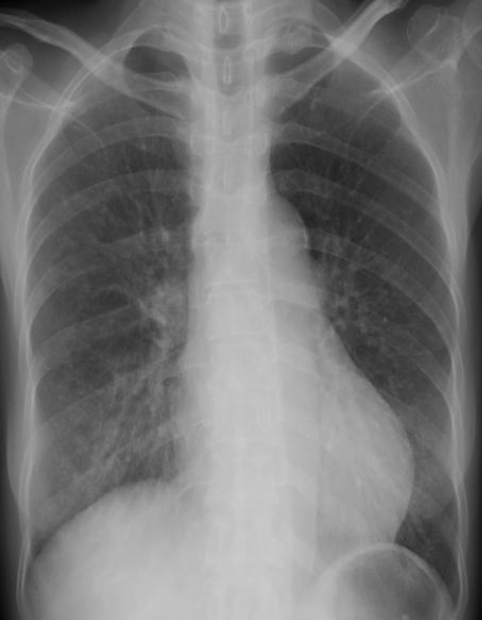
A 55-year-old woman presented with fever for 3 days. She had worked as a car saleswoman in Pennsylvania and had arrived in Seoul the day before admission. She had suffered from RA for about 20 years and had been treated with a regimen including prednisolone, methotrexate, and etanercept for 2 years. She had received subcutaneous etanercept at dosages of 25 mg twice a week. She had noticed progressive fatigue, malaise, anorexia, oral bleeding, and a weight loss of 10 kg within the previous 3 months. Upon admission, her body temperature was 37.5Ōäā, blood pressure was 120/80 mmHg, pulse rate was 104 beats per minute, and respiratory rate was 18 breaths per minute. On physical examination, she appeared chronically ill with a pale conjunctiva, oral ulcer, and advanced multiple symmetrical joint deformities of both hands. Her chest examination was notable for fine rales on both lower lung fields, but no murmur was noted. All other findings were unremarkable. Laboratory findings revealed hemoglobin at 7.7 g/dL, a platelet count of 40,000/mm3, and a white blood cell count of 3,000/mm3 with 65% granulocytes, 26% lymphocytes, and 18.3% monocytes. Liver function tests revealed aspartate aminotransferase at 447 IU/L, alanine aminotransferase at 113 IU/L, and albumin at 2.4 g/dL. Her rheumatoid factor was 2,180 IU/mL. Antimycoplasma and anti-HIV antibodies were negative. Chest radiography showed poorly-defined nodular opacities in both lower lung zones (Figure 1A). A CT scan of the abdomen revealed enlarged lymph nodes in the para-aortic area (Figure 1B). On hospital day 4, she complained of dyspnea, and her oxygen saturation dropped below 80% while she was breathing 100% oxygen. She was intubated and transferred to the intensive care unit. Pulmonary hemorrhage was suspected, and chest radiography showed diffuse bilateral pulmonary consolidation in both lungs (Figure 2A). A CT scan of the chest obtained at the same time showed diffuse consolidation that was strongly suspicious for pulmonary hemorrhage combined with pneumonia (Figure 2B). Transthoracic echocardiography revealed minimal pericardial effusion with diffuse hypokinesia and a left ventricular (LV) ejection fraction of 36%. A bone marrow examination showed hypocellular marrow with no maturation arrest. We performed a bronchoscopy that showed mucosal injection and fold thickening, but could not find a definite focus of bleeding. The blood and endotracheal cultures for bacteria, fungus and mycobacteria were negative. Intravenous immunoglobulin (35 g/day) was given for sepsis, and solucortef (50 mg/day) was maintained for RA. Treatment with azithromycin, ceftriaxone and amikacin was started, but because her condition deteriorated, these were changed to meropenem and ciprofloxacin on hospital day 10. Her condition improved, with chest radiograph showing complete resolution of previous lesions by hospital day 40 (Figure 3). Follow-up echocardiography showed normal LV systolic function with an ejection fraction of 70% without hypokinesia. The result of endotracheal aspiration for viruses was positive for adenovirus, which was confirmed by PCR.
After discharge, she maintained oral medication including prednisolone at 10 mg per day, celecoxib at 200 mg per day, hydroxychloroquine sulfate at 300 mg per day and methotrexate at 7.5 mg per week through an outpatient clinic without complications. She remained well without aggravating arthralgia.
TNF-╬▒ is a cytokine that plays a critical role in the regulation of inflammatory processes due to infection5). Early in the infectious process, TNF-╬▒ promotes the influx of cells to the injured area to counter the inciting agent, while it subsequently helps to limit the extent of damage by inducing apoptosis and maintaining granuloma formation. These essential functions may be compromised by inhibition of TNF-╬▒ via anti-TNF-╬▒ blockers, so that patients who receive anti-TNF-╬▒ blocking therapy are vulnerable to new or latent infection6).
The spectrum of pathogens causing invasive disease in patients receiving TNF-╬▒ blockade therapy ranges from bacteria to more opportunistic organisms such as Mycobacterium tuberculosis, cryptococcus and aspergillus6). A recent review of reports from 1998 to 2002 revealed that the incidence of granulomatous infections was 74 per 100,000 using etanercept7).
M. tuberculosis is the most frequently reported infection in patients treated with etanercept, with a rate of 35 per 100,000. This is much lower than the rate of 144 per 100,000 in infliximab-treated7). The median time to develop the infection of M. tuberculosis was reportedly 12 weeks in patients treated with infliximab, whereas it was 46 weeks in those treated with etanercept5).
In clinical trials involving etanercept treatment in RA, infections of the upper respiratory tract were the most commonly reported, whereas serious infections requiring hospitalization were rare and did not increase in frequency during treatment8). However, in a more recent study, serious and non-serious respiratory tract infections were significantly more common in patients receiving etanercept than in patients treated with conventional disease-modifying antirheumatic drugs (7.04 versus 1.75 per 100 patient-years)9).
As for viral infections, TNF is integral to the immune response to many pathogens, including respiratory viruses. Binding of TNF cytokines to their receptors initiates several signaling pathways culminating in the activation of transcription factors, cysteine proteases, which results in apoptosis, and cytosolic phospholipase A2, an enzyme responsible for the production of inflammatory mediators, which are involved in cytolysis by TNF10). Etanercept may allow benign respiratory viral infections to progress through inhibition of TNF.
Adenoviruses are non-enveloped, icosahedral DNA viruses that cause a variety of clinical syndromes. The majority of these infections are self-limited, with disseminated disease occurring rarely in immunocompetent patients11). Most severe or disseminated diseases tend to occur exclusively in immunocompromised patients. Involvement of the lung, liver, pancreas, heart, colon and central nervous system has been reported. Possible risk factors in these disseminated cases include the underlying medical condition, prolonged hospitalization, invasive procedures, broad-spectrum antibiotics and immunosuppressive drugs11). Adenovirus is thought to cause approximately 10% of cases of respiratory disease in children. Four (1.3%) of 317 Korean adult patients with community- acquired pneumonia were diagnosed with adenoviral pneumonia by indirect immunofluorescence staining or culture12). Adenoviruses can also infect the mucosal endothelium, as well as the epithelium11), which can lead to life-threatening hemorrhage, as was observed in this case. Furthermore, sudden decreased myocardial function without identifiable precipitating factors also developed during hospitalization, and function fully recovered without sequelae. This can be attributed to adenoviral myocarditis. Since TNF-╬▒ exerts widespread biological effects on immune cells, it is not surprising that TNF inhibition results in a decreased ability to control infection in both animal models and humans. In this case, adenoviral pneumonia with myocarditis was recovered after stopping etanercept treatment.
We conclude that all patients should be thoroughly assessed for the presence of any possible risk factors for infection prior to commencement of etanercept therapy. We recommend close monitoring for any infections during the etanercept treatment and suggest that discontinuation of therapy may be indicated during the development of infection7). Further study of the relationship between etanercept therapy and respiratory viral infections may be warranted.
References
1. Peno-Green L, Lluberas G, Kingsley T, Brantley S. Lung injury linked to etanercept therapy. Chest 2002. 122:1858ŌĆō1860PMID : 12426295.


2. Moreland LW, Cohen SB, Baumgartner SW, Tindall EA, Bulpitt K, Martin R, Weinblatt M, Taborn J, Weaver A, Burge DJ, Schiff MH. Long-term safety and efficacy of etanercept in patients with rheumatoid arthritis. J Rheumatol 2001. 28:1238ŌĆō1244PMID : 11409115.

3. Phillips K, Husni ME, Karlson EW, Coblyn JS. Experience with etanercept in an academic medical center: are infection rates increased? Arthritis Rheum 2002. 47:17ŌĆō21PMID : 11932873.


4. Smith D, Letendre S. Viral pneumonia as a serious complication of etanercept therapy. Ann Intern Med 2002. 136:174. PMID : 11790076.

5. Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum 2003. 48:3013ŌĆō3022PMID : 14613261.


6. Cunnane G, Doran M, Bresnihan B. Infections and biological therapy in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2003. 17:345ŌĆō363PMID : 12787529.


7. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004. 38:1261ŌĆō1265PMID : 15127338.


8. Moreland LW. Drugs that block tumor necrosis factor: experience in patients with rheumatoid arthritis. Pharmacoeconomics 2004. 22(Suppl):39ŌĆō53PMID : 15157003.


9. Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, Gromnica-Ihle E, Antoni C, Herzer P, Kekow J, Schneider M, Zink A. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 2005. 52:3403ŌĆō3412PMID : 16255017.


10. Burgert HG, Blusch JH. Immunomodulatory functions encoded by the E3 transcription unit of adenoviruses. Virus Genes 2000. 21:13ŌĆō25PMID : 11022786.


11. Pham TT, Burchette JL Jr, Hale LP. Fatal disseminated adenovirus infections in immunocompromised patients. Am J Clin Pathol 2003. 120:575ŌĆō583PMID : 14560569.


12. Kim JH, Kwak YH, Na BK, Lee JY, Shin GC, Jung HS, Hong JY, Oh MD, Cheong HJ, Kim MJ, Pai HJ, Kim YR, Shin WS, Kang JM, Woo JH, Uh ST, Lee HJ. Viral etiology of community-acquired pneumonia in Korean adults. Korean J Infect Dis 2001. 33:8ŌĆō14.
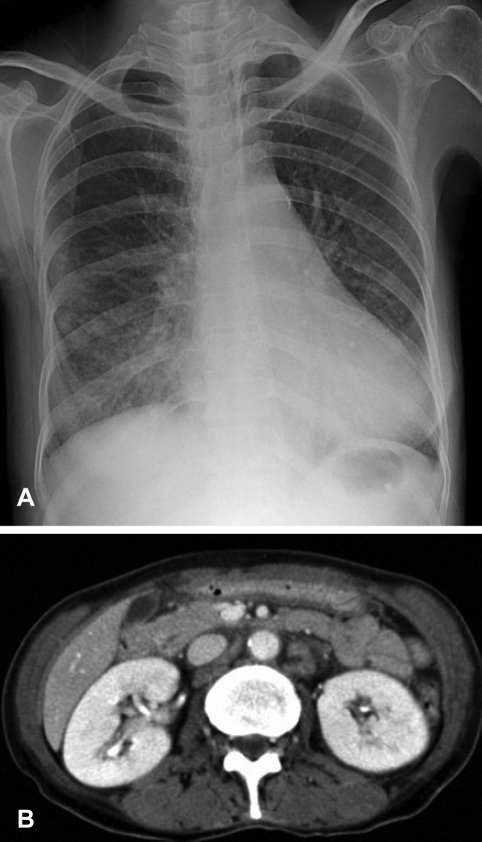
Figure┬Ā1
(A) An initial chest radiograph shows poorly-defined nodular opacities and consolidation in both lower lung zones. (B) A contrast-enhanced abdominal CT scan reveals enlarged lymph nodes with central attenuation in the para-aortic area.
Figure┬Ā2
(A) A chest radiograph obtained on hospital day 4 shows diffuse bilateral pulmonary consolidation in both lungs that is consistent with diffuse lung injury. (B) A chest CT scan also obtained on hospital day 4 shows diffuse consolidation and ground-glass attenuation in both lungs, as well as small bilateral pleural effusions. Enlarged lymph nodes with central attenuation are evident in the right paratracheal area.

-
METRICS

- Related articles
-
Perioperative and anesthetic management of patients with rheumatoid arthritis2022 July;37(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


